Figure 4.
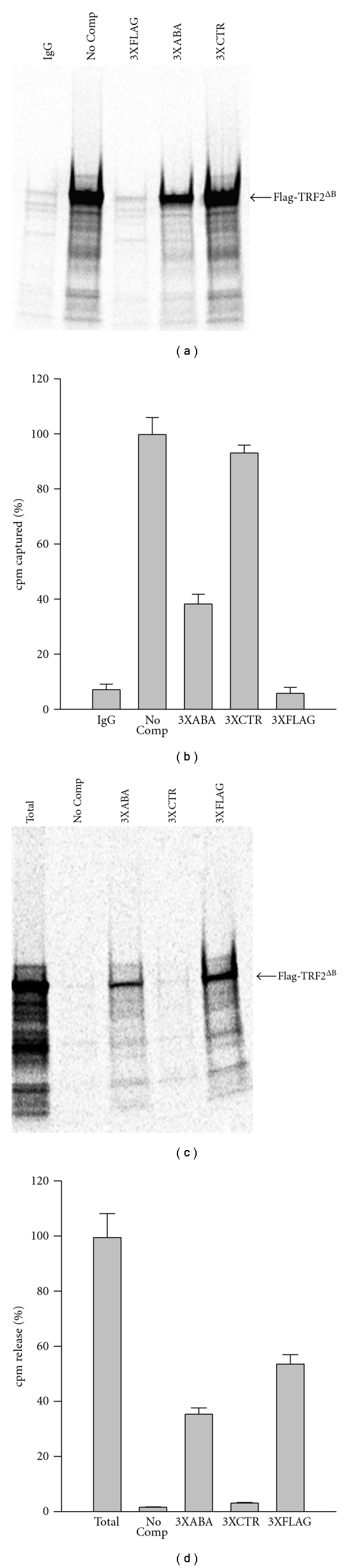
3XABA oligonucleotide blocks the interaction of Flag-tagged proteins with the M2 antibody. (a)-(b) The 3XABA oligo blocks the binding of Flag-TRF2ΔB to M2-coated beads. Magnetic beads coated with the M2 antibody were incubated in the absence (No Comp) or presence of the indicated competitor (3XABA, 3XCTR, 3XFLAG). In vitro translated [35S]-labeled Flag-TRF2ΔB was then added and the amount captured by the beads was determined by SDS-PAGE electrophoresis and exposure to a PhophorImager cassette (a). In a second experiment done in triplicate, the amount of [35S]-labeled protein captured was counted by scintillation (b). The amount of [35S]-labeled protein captured in the absence of competitor (No Comp) was arbitrarily set to 100%. In both experiments, beads coated with normal mouse IgG were included as negative control for the capture (IgG). Data represent the mean ± S.D. (n = 3). (c)-(d) The 3XABA oligo elutes the Flag-TRF2ΔB proteins already bound to M2-coated beads. The [35S]-Flag-TRF2ΔB protein was first captured by magnetic beads coated with the M2 antibody. The beads were then incubated in the absence (No comp) or presence of the indicated competitor (3XABA, 3XCTR, 3XFLAG). The amount of [35S]-Flag-TRF2ΔB released was determined by SDS-PAGE electrophoresis and exposure to a PhophorImager cassette (c). In a second experiment done in triplicate, the amount of [35S]-labeled protein released was counted by scintillation (d). The amount of [35S]-labeled protein released by the boiling (total) was arbitrarily set to 100%. In both experiments, beads boiled to release to all of the captured [35S]-labeled protein were included as positive control for the elution (Total). Data represent the mean ± S.D. (n = 3).
