Abstract
Deciphering the complexities of human β cell physiology is critical to our understanding of the pathophysiology behind both type 1 and type 2 diabetes. One way to do this is to study individuals with congenital hyperinsulinism (CHI), a rare genetic disease characterized by dysregulation of insulin secretion resulting in hypoglycemia. In this issue of the JCI, Henquin et al. report in vitro studies of pancreatic tissue obtained from CHI patients during therapeutic pancreatectomy that have yielded exciting new insights into human β cell physiology. The data validate and extend observations made in model organisms.
β Cell dysfunction lies at the center of both major forms of diabetes, the incidence of which is increasing at an alarming rate in both the developed and the developing world (1). In type 1 diabetes mellitus (T1DM), autoimmune β cell destruction results in complete insulin deficiency, whereas in type 2 diabetes mellitus (T2DM), subtle defects in β cell functional mass result in progressive disease. Studies using animal models and cell lines from various sources have yielded invaluable information that has allowed us to better understand the processes responsible for β cell function, dysfunction, replication, and survival. However, since the model systems may not always accurately reflect in vivo human physiology, validation studies in humans are crucial. The crunch comes, however, in that performing such studies is at best difficult and often impossible. Genetic manipulation in vivo, a hugely powerful tool in animal-based research, is obviously impossible in humans, and in vitro genetic manipulations of primary human tissues are difficult and in themselves fraught with pitfalls.
One way to overcome some of these problems is to identify and study individuals with naturally occurring genetic mutations. These cases are rare and often difficult to study, and the results may be difficult to interpret. With animal models, experiments can be performed in genetically identical animals under controlled conditions. In human studies, however, each patient typically has a different mutation and studies are done at different ages, under different experimental circumstances, and after different environmental exposures. Patients, or their guardians, frequently do not agree to perform detailed in vivo studies unless they are clinically required, and relevant tissues are often not available for study. These issues make such studies infinitely more difficult, yet because of the limitations of alternative approaches, they are absolutely critical to advancing our understanding of human (as opposed to rodent) physiology.
In this issue of the JCI, Henquin and colleagues describe their attempts to decipher the complexities of human β cell physiology through detailed in vitro physiologic analysis of tissues obtained from 24 patients with congenital hyperinsulinism (CHI) (2), a rare genetic disorder that is essentially the opposite of diabetes, since it is characterized by hypoglycemia, not hyperglycemia (3). CHI is no less relevant to β cell biology than the monogenic forms of diabetes, which are extremely difficult to study, since pancreatic tissue from individuals with these conditions is almost never available. As the most comprehensive study to date using pancreatic tissue obtained from patients with genetically defined CHI, the work of Henquin and colleagues represents a milestone in β cell research (2).
CHI: the basics
CHI is a clinically and genetically heterogeneous disorder typically diagnosed in the newborn period (3). Prior to the discovery of the precise genetic etiology of the most common genetic form of this disease in 1995 (4), the pathophysiology and clinical features were hotly debated, and the clinical syndrome was referred to by different descriptive names. These included nesidioblastosis, which described the histologic picture that was subsequently shown to be nonspecific (5); β cell dysmaturation syndrome, since some histologic and functional characteristics of the disease are reminiscent of the fetal pancreas (6); persistent hyperinsulinism of infancy (PHHI); hyperinsulinemic hypoglycemia, familial (HHF); and CHI. The latter, used by Henquin and colleagues (2), seems to be most popular, although the OMIM website uses HHF (OMIM 256450).
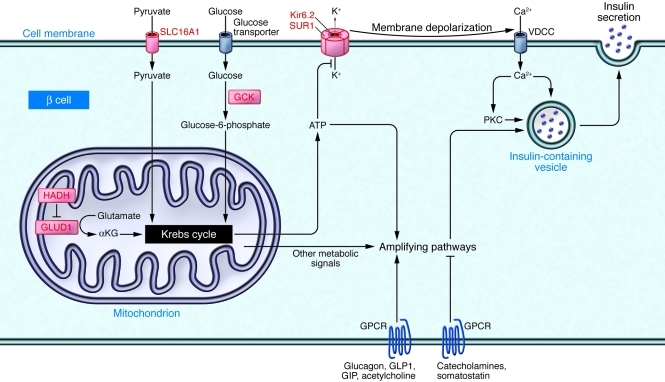
Once thought to be a single entity, it is now known that CHI can be caused by mutations in at least six different genes (ABCC8, KCNJ11, GCK, GLUD1, HADH, and SLC16A1), each of which affects the β cell differently (Figure 1), resulting in somewhat divergent phenotypes. In many CHI patients, the etiology is still unknown, suggesting that mutations in other genes can cause a similar phenotype.
Figure 1. Schematic representation of the major genetic etiologies of CHI.
Some of the major pathways regulating insulin secretion are shown. The proteins encoded by genes mutated in different forms of CHI are shown in red. Mutations in either ABCC8 (which encodes SUR1) or KCNJ11 (which encodes Kir6.2) result in defective KATP channel activity and continuous membrane depolarization regardless of glucose levels. Activating mutations in glucokinase (GCK) result in increased glucose metabolism at low circulating glucose levels, resulting in a decreased threshold for glucose-stimulated insulin secretion and thus inappropriate insulin secretion in the presence of hypoglycemia (18). Glutamate dehydrogenase 1 (GLUD1) mutations result in increased conversion of glutamate to α-ketoglutarate (αKG), thereby increasing ATP production and insulin secretion (19). Solute carrier family 16, member 1 (SLC16A1) promoter mutations result in inappropriate expression of this transporter in β cells, allowing pyruvate and lactate to enter the cell. Pyruvate can then enter the Krebs cycle, thus increasing ATP production and insulin secretion. Patients with mutations in this gene have a unique form of CHI characterized by exercise-induced hypoglycemia (20). Inactivating mutations in hydroxyacyl-CoA dehydrogenase (HADH) also cause CHI and have been shown to do so by regulating insulin secretion in a KATP channel–independent fashion (21) and by preventing HADH-mediated inhibition of GLUD1 in the β cell (22). Glucagon-like peptide 1 (GLP1), glucose-dependent insulinotrophic peptide (GIP), glucagon, somatostatin, and other signals not shown in this simplified diagram regulate critical amplifying pathways downstream from the CHI-related proteins.
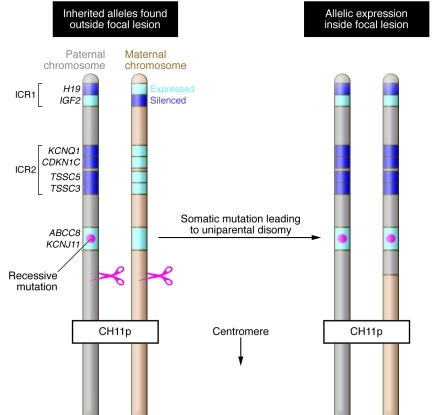
The most common form of CHI, and the one studied by Henquin and colleagues (2), is caused by inactivating mutations in either of the two genes encoding the subunits of the β cell KATP channel — ABCC8, which encodes sulfonylurea receptor 1 (SUR1), and KCNJ11, which encodes the inward-rectifier potassium channel (Kir6.2). Channel inactivation in these patients results in membrane depolarization and opening of voltage-gated calcium channels, which triggers inappropriate insulin exocytosis. In some patients, abnormal β cells are localized to a discrete focus consisting of a conglomerate of huge, metabolically active islets, with β cells outside of this region showing small nuclei and decreased cytoplasm, suggestive of suppressed function. The unique genetic etiology of this focal form of disease was defined in 1996 (7, 8) and consists of a paternally inherited recessive KATP gene mutation and somatic loss of heterozygosity in a β cell precursor (Figure 2). Such patients can be cured by resection of the lesion. All other genetic mutations in ABCC8 and KCNJ11 result in diffuse forms of CHI, in which all β cells are abnormal. Individuals with this condition often require subtotal pancreatectomy, which is frequently followed by either persistent hypoglycemia or diabetes, depending on the extent of the pancreatectomy and the severity of the mutation.
Figure 2. Schematic representation of the molecular defect resulting in focal CHI.
On the left is shown a schematic representation of the paternal and maternal copies of the distal short arm of chromosome 11, inherited by the fetus at risk for focal CHI and present in all cells except those within the focal lesion. A single ABCC8 or KCNJ11 recessive mutation is inherited on the paternal allele (red dot). Distal to the ABCC8/KCNJ11 locus, there are two imprinted regions. The relevant genes are shown, and the expressed allele for each is shown in light blue. During fetal development, a somatic mutation occurs in a β cell precursor, which results in loss of the maternally inherited allele and duplication of the paternally inherited allele, as depicted on the right. In this cell and all of its progeny, both ABCC8 or KCNJ11 alleles are mutated, resulting in insulin hypersecretion. In addition, two copies of growth-promoting genes such as IGF2 are expressed, while growth-inhibiting genes such as H19 and CDKN1C are not expressed at all. The result is a proliferating lesion consisting of β cells lacking a functioning KATP channel and thus hypersecreting insulin at low glucose levels.
Functional defects in CHI β cells: prior state of the art
The study by Henquin and colleagues (2), which characterized the in vitro kinetics of insulin secretion by pancreatic fragments obtained at the time of therapeutic pancreatectomy from six patients with diffuse CHI and 18 patients with focal CHI, expands upon previous in vivo and in vitro findings in patients with CHI. Although multiple studies have described the electrophysiological characteristics of affected β cells (9, 10), only a few have examined in vitro insulin secretion in response to secretagogues, and all of these were of limited scope due to scarcity of tissue (11–13). Aynsley-Green demonstrated some increase in insulin secretion by β cells from CHI patients when glucose levels were increased from 0 to 4 mmol, but no further increase at higher concentrations (11). About a decade later, Kaiser and colleagues used static incubations to study pancreatic tissue from five patients with CHI, demonstrating that while stimulators of cAMP production and modulators of the PI3K/PKC pathway stimulated insulin release, glucose did not (12). Secretion was shown to be calcium dependent and partially suppressed by epinephrine or somatostatin. Although this study was performed before the discovery of the genes encoding the KATP channel subunits, one patient was subsequently found to be homozygous for the ABCC8 p.R836* mutation, while three others, all from the same large Arab family, were shown to be homozygous for the ABCC8 c.950delC mutation. Subsequently, Otonkoski and colleagues reported poor responsiveness to glucose and partial suppression with somatostatin in pancreases from six patients with CHI, using human fetal islets for comparison (13).
Functional defects in CHI β cells: novel findings
All previous in vitro studies of CHI-derived pancreatic islets lacked optimal controls, since age-matched normal human islets are not available. Henquin and colleagues solved this problem by using β cells isolated from outside focal lesions as controls (2). Although β cells outside the focal lesion are functionally suppressed in vivo due to increased insulin secretion and hypoglycemia, being heterozygous for recessive mutations, they are expected to function normally once outside the patient. Indeed, Henquin and colleagues show that a short period of incubation in stimulatory glucose concentrations is sufficient to restore their responsiveness (2). That being said, the authors are correct in advising caution, since this recovery may not be complete. Using these control islets and a sensitive perifusion system that allowed detailed analysis of secretory dynamics, Henquin and colleagues demonstrated conclusively that CHI islets hypersecrete insulin even at very low glucose levels. Additional findings, such as minimal or no insulin response to glucose or the KATP channel blocker tolbutamide, marked response to stimulators of cAMP, and variable responses to different amino acids were consistent with previous reports and current models of the control of insulin secretion. The finding that β cells from both patients with focal CHI and those with diffuse CHI respond similarly demonstrates that, although the imprinted genes in the distal arm of chromosome 11 may affect β cell replication (14), they do not affect insulin secretion. This finding is novel, albeit expected, since focal CHI and diffuse CHI are clinically similar (15).
In addition to the aforementioned findings, which might have been predicted from previous work, Henquin and colleagues made some unexpected observations (2). Acute elevation of glucose from 1 to 15 mmol/l resulted in transiently increased insulin secretion in some CHI islet preparations. This was unexpected, since this acute insulin response to glucose is thought to be mediated through the KATP channel. Also unexpectedly, insulin secretion decreased during high glucose infusion in some studies, primarily those in which forskolin, which raises cAMP levels, was administered simultaneously. That the transient peak insulin secretion is not mediated through the KATP channel is not entirely unexpected, since some children with CHI do show a brief increase in insulin secretion following glucose stimulation (16). This could be due to further membrane depolarization; however, this seems unlikely since electrophysiological studies in similar patients do not show a glucose effect on β cell calcium currents (9). Alternatively, glucose may be transiently acting downstream of membrane depolarization, stimulating amplifying pathways. The decrease in insulin secretion during perifusion with 15 mmol glucose is also unexpected, since continuous exposure to high glucose levels is expected to result in continuously increased insulin secretion, mediated largely by KATP channel–independent pathways. This surprising observation is not explained by Henquin and colleagues (2), but may reflect idiosyncrasies of the experimental system, since a similar decrease was less evident in glucose ramp experiments or in the absence of forskolin.
Another unexpected finding was the paradoxical response to diazoxide seen in some, but not all, patients. As a KATP channel activator, diazoxide would be expected to be without effect in patients with either ABCC8 or KCNJ11 mutations, as these mutations were thought to either result in permanently closed KATP channels or to entirely prevent protein expression and/or insertion into the plasma membrane. Interestingly, Henquin and colleagues saw a response to diazoxide in one of three patients with mutations that introduced stop codons before the region of ABCC8 that encodes the first nucleotide-binding domain (2), far proximal to the putative potassium channel opener binding site (17). This suggests that the paradoxical response to diazoxide is likely to be an off-target effect. A similar paradoxical response was seen with pinacidil, a KATP channel opener with different channel specificity. While this speaks against an off-target effect, it does not entirely eliminate this possibility. Unfortunately, future detailed studies in human CHI β cells are unlikely, given how difficult it is to obtain appropriate samples. Although, as Henquin and colleagues state (2), this paradoxical response has not been reported in animal models of CHI, this can be tested directly using identical experimental protocols. Furthermore, it is not known whether a similar response is present in vivo, since acute response studies have not been reported in CHI patients with KATP channel mutations.
Conclusion
Given the rarity of CHI and the technical difficulty in obtaining sufficient normal and abnormal tissue, it is not surprising that it took Henquin and colleagues ten years to accumulate sufficient data for their publication (2). The major findings reported here confirm previous findings in humans and in animal models. The novel and unexpected discoveries are not entirely explained, and additional studies, presumably using animal models or in vivo studies in humans, are needed to arrive at a satisfying mechanistic explanation. Nevertheless, this experimental tour de force represents a milestone in β cell research, as it made use of a human inherited condition to validate and expand on models of β cell function largely established in rodent models.
Acknowledgments
I would like to thank Yuval Dor for reviewing the manuscript and providing very helpful comments and suggestions.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(10):3821–3825. doi:10.1172/JCI60002.
See the related article beginning on page 3932.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Henquin J-C, et al. In vitro insulin secretion by pancreatic tissue from infants with diazoxide-resistant congenital hyperinsulinism deviates from model predictions. J Clin Invest. 2011;121(10):3932–3942. doi: 10.1172/JCI58400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaser B, Landau H, Permutt MA. Neonatal hyperinsulinism. Trends Endocrinol Metab. 1999;10(2):55–61. doi: 10.1016/s1043-2760(98)00102-7. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PM, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268(5209):426–429. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- 5.Rahier J. Relevance of endocrine pancreas nesidioblastosis to hyperinsulinemic hypoglycemia. Diabetes Care. 1989;12(2):164–166. doi: 10.2337/diacare.12.2.164. [DOI] [PubMed] [Google Scholar]
- 6.Heitz PU, Kloppel G, Hacki WH, Polak JM, Pearse AG. Nesidioblastosis: the pathologic basis of persistent hyperinsulinemic hypoglycemia in infants. Morphologic and quantitative analysis of seven cases based on specific immunostaining and electron microscopy. Diabetes. 1977;26(7):632–642. doi: 10.2337/diabetes.26.7.632. [DOI] [PubMed] [Google Scholar]
- 7.Fournet JC, et al. Loss of imprinted genes and paternal SUR1 mutations lead to hyperinsulinism in focal adenomatous hyperplasia. Ann Endocrinol (Paris). 1998;59(6):485–491. [PubMed] [Google Scholar]
- 8.Ryan FD, et al. Hyperinsulinism: The molecular aetiology of focal disease. Arch Dis Child. 1998;79(5):445–447. doi: 10.1136/adc.79.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straub SG, et al. Hyperinsulinism of infancy: the regulated release of insulin by KATP channel-independent pathways. Diabetes. 2001;50(2):329–339. doi: 10.2337/diabetes.50.2.329. [DOI] [PubMed] [Google Scholar]
- 10.Kane C, et al. Loss of functional KATP channels in pancreatic β-cells causes persistent hyperinsulinemic hypoglycemia of infancy. Nat Med. 1996;2(12):1344–1347. doi: 10.1038/nm1296-1344. [DOI] [PubMed] [Google Scholar]
- 11.Aynsley-Green A. Nesidioblastosis of the pancreas in infancy. Dev Med Child Neurol. 1981;23(3):372–379. [PubMed] [Google Scholar]
- 12.Kaiser N, et al. Regulation of insulin release in persistent hyperinsulinaemic hypoglycaemia of infancy studied in long-term culture of pancreatic tissue. Diabetologia. 1990;33(8):482–488. doi: 10.1007/BF00405110. [DOI] [PubMed] [Google Scholar]
- 13.Otonkoski T, Andersson S, Simell O. Somatostatin regulation of β-cell function in the normal human fetuses and in neonates with persistent hyperinsulinemic hypoglycemia. J Clin Endocrinol Metab. 1993;76(1):184–188. doi: 10.1210/jcem.76.1.8093619. [DOI] [PubMed] [Google Scholar]
- 14.Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. β-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes. 2000;49(8):1325–1333. doi: 10.2337/diabetes.49.8.1325. [DOI] [PubMed] [Google Scholar]
- 15.de Lonlay P, et al. Heterogeneity of persistent hyperinsulinaemic hypoglycaemia. A series of 175 cases. Eur J Pediatr. 2002;161(1):37–48. doi: 10.1007/s004310100847. [DOI] [PubMed] [Google Scholar]
- 16.Grimberg A, et al. Dysregulation of insulin secretion in children with congenital hyperinsulinism due to sulfonylurea receptor mutations. Diabetes. 2001;50(2):322–328. doi: 10.2337/diabetes.50.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhde I, Toman A, Gross I, Schwanstecher C, Schwanstecher M. Identification of the potassium channel opener site on sulfonylurea receptors. J Biol Chem. 1999;274(40):28079–28082. doi: 10.1074/jbc.274.40.28079. [DOI] [PubMed] [Google Scholar]
- 18.Glaser B, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med. 1998;338(4):226–230. doi: 10.1056/NEJM199801223380404. [DOI] [PubMed] [Google Scholar]
- 19.Stanley CA, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998;338(19):1352–1357. doi: 10.1056/NEJM199805073381904. [DOI] [PubMed] [Google Scholar]
- 20.Otonkoski T, et al. Physical exercise-induced hypoglycemia caused by failed silencing of monocarboxylate transporter 1 in pancreatic β cells. Am J Hum Genet. 2007;81(3):467–474. doi: 10.1086/520960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy OT, et al. Functional genomics of the β-cell: short-chain 3-hydroxyacyl-coenzyme A dehydrogenase regulates insulin secretion independent of K+ currents. Mol Endocrinol. 2007;21(3):765–773. doi: 10.1210/me.2006-0411. [DOI] [PubMed] [Google Scholar]
- 22.Li C, et al. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. . J Biol Chem. 2010;285(41):31806–31818. doi: 10.1074/jbc.M110.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]