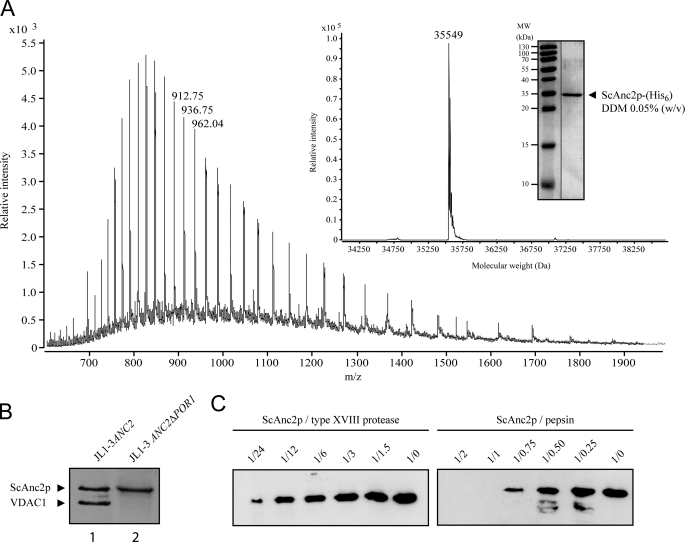
FIGURE 1.
Analysis of the ScAnc2p primary amino acid sequence and proteolysis in HDX-compatible conditions. A, LC ESI-ToF-MS analysis of ScAnc2p-His6 in 0.05% (w/v) n-dodecyl-β-d-maltoside (DDM) solution is shown. Inset, shown is a deconvoluted spectrum of the carrier purified from crude mitochondrial extracts from JL1–3 ANC2(His6) strain. In the gel, proteins were analyzed by SDS-PAGE (12.5% acrylamide) and revealed by Coomassie Blue staining. B, ScAnc2p purification from crude mitochondrial extracts of JL1–3ANC2 (lane 1) or JL1–3Δpor1-ANC2 (lane 2) strains were analyzed by SDS-PAGE as for A. C, ScAnc2p proteolysis is shown. Lysates from pepsin and type XVIII digestion were analyzed by SDS-PAGE as for A. Fragments were revealed by Western blot with anti-SDS-ScAnc2p. Digestion was performed in acidic buffer for 2 min at 4 °C, with 2 μg of ScAnc2p at a substrate/protease ratio between 1:0.25 and 1:2 (w/w) for pepsin or between 1:1.5 and 1:24 (w/w) for type XVIII protease.