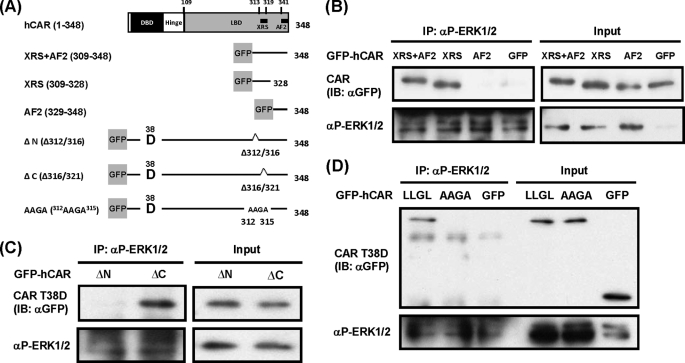
FIGURE 3.
Interaction between p-ERK1/2 and the XRS of hCAR T38D. A, schematic representation of the domains and motifs of the CAR molecule and GFP-tagged deletion constructs. XRS+AF2 contains the XRS and AF2 domain. XRS and AF2 contain only the XRS or AF2 domain. ΔN has an internal deletion of the N-terminal half of XRS (Δ312/316) within the context of GFP-hCAR T38D, whereas the C-terminal half of XRS (Δ316/321) was internally deleted in ΔC. In the AAGA construct, three leucines (residues 312, 313, and 315) were simultaneously mutated to alanines within the context of the GFP-hCAR T38D mutant. DBD, DNA-binding domain; LBD, ligand-binding domain. B–D, these GFP-tagged mutants were expressed in Huh-7 cells for 24 h, and cytosolic extracts were prepared and incubated with an anti-p-ERK1/2 antibody. The resultant precipitates were analyzed by Western blotting with an anti-GFP antibody for co-immunoprecipitation (IP) of these hCAR mutants with p-ERK1/2. Cytosolic extracts were also blotted with an anti-GFP antibody to confirm the expression of CAR mutants (2% input). IB, immunoblot.