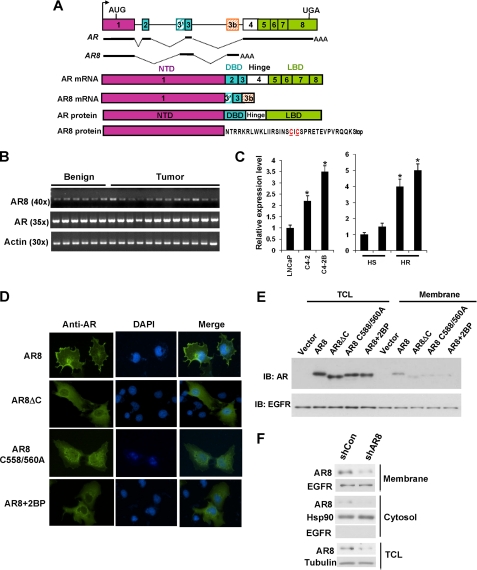
FIGURE 1.
Cloning and expression of membrane-bound AR8. A, schematic structure of human AR splice variants. The hatched cassettes indicate the cryptic exons. Solid thick lines represent the transcribed exon sequences. The AR8 unique amino acid sequence is shown, and putative palmitoylation sites are in red and underlined. B, expression of AR8 in human prostate tissues. Total RNA was isolated from 6 benign and 12 malignant human prostate tissues and subjected to reverse transcription-PCR. The primer sets used to amplify AR- or AR8-specific transcripts were described under “Experimental Procedures.” C, relative expression levels of AR8 in LNCaP, C4-2, and C4-2B were quantified using real-time PCR (left). Their expression in two pairs of CW22R xenograft tumors derived from intact (HS) and castrated (HR) male mice were also quantified (right). *, p < 0.05. Error bars, S.D. D, plasma membrane localization of AR8. COS-1 cells were transfected with AR8 or AR8 mutants. Some cells were treated with 25 μm 2-bromopalmitic acid (2BP) for 16 h as indicated. Cells were then subjected to immunofluorescence staining with anti-AR (N20) antibody. Nucleus was visualized using DAPI staining. E, effects of AR8 mutation on its membrane localization. COS-1 cells were transfected with the indicated constructs. Membrane fractionation was carried out using the Eukaryotic Membrane Protein Extraction kit (Pierce). AR protein present in membrane fractions and total lysates were detected by Western blotting (IB) with an anti-AR antibody. EGFR served as a loading control. F, CWR-R1 cells were infected with lentivirus encoding the shRNA for AR8 (shAR8) or the scrambled control (shCon). At 48 h after infection membrane fractionation was carried out as above. The level of AR8 protein in the membrane or cytosol fraction as well as in the total lysates was detected by Western blotting with the anti-AR8 antibody. Tubulin serves as a loading control and anti-Hsp90, anti-EGFR to assign the majorities of the marker proteins to their expected cytoplasmic, membrane fractions respectively.