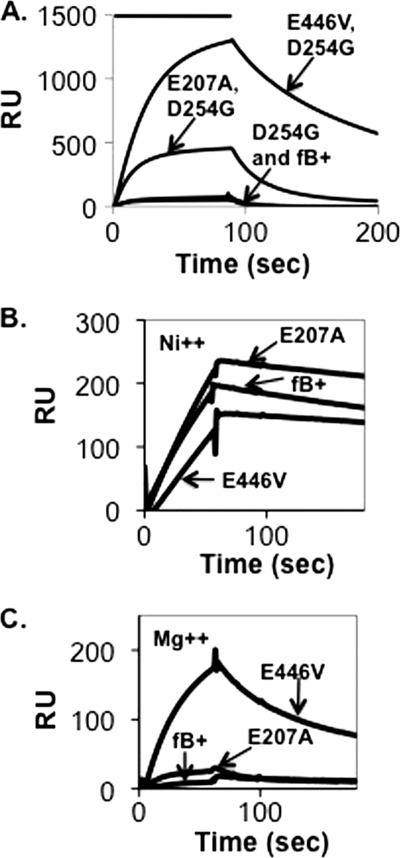
FIGURE 5.
Assembly and dissociation of C3bB complexes on a C3b-coated biosensor surface. A, C3b (5942 response units (RU)) was covalently attached to a biosensor surface and treated for 90 s with 10 μg/ml wild-type recombinant factor B, factor B D254G, factor B E446V/D254G, or factor B E207A/D254G in Mg2+ HEPES buffer followed by buffer alone. The resulting profiles were aligned at t = 0. The peak of the E446V/D254G profile represents complexes formed with 44% of the surface-bound C3b. B, C3b (7205 response units) was covalently attached to a biosensor surface and treated for 90 s with 1 μg/ml wild-type recombinant factor B or factor B E446V in Ni2+ HEPES buffer followed by buffer alone. The peak of the E446V profile represents complexes formed with 10% of the surface-bound C3b. The resulting profiles were aligned at t = 0. C, the C3b-coated biosensor shown in A was treated for 90 s with 1 μg/ml wild-type recombinant factor B or factor B E446V as above but in Mg2+ HEPES buffer.