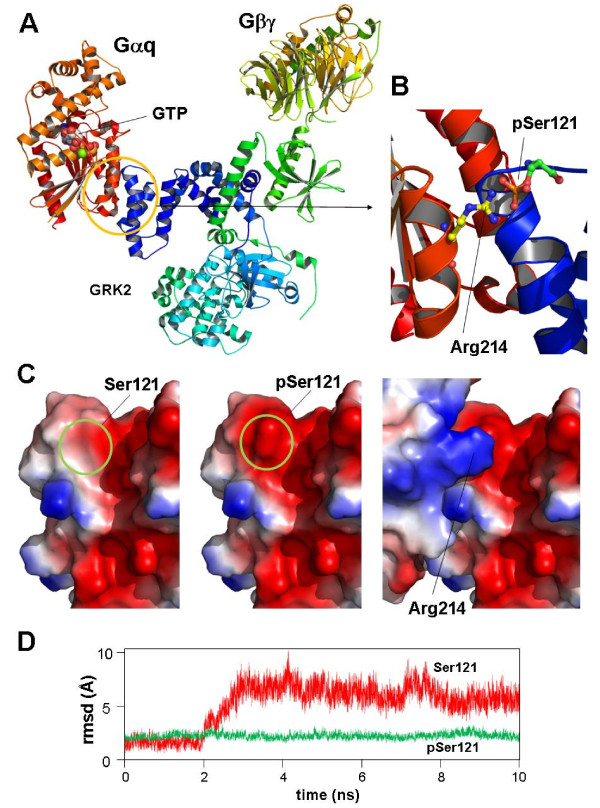
Figure 3.
Case study. Analysis of the structural interactions of GRK2 [Swiss-Prot: P21146], Gαq [Swiss-Prot: P21279] and Gβγ proteins [Swiss-Prot: P62871and Swiss-Prot: P63212] according to the crystallized structure of the macromolecular complex [PDB: 2BCJ]. A. Crystallized structure of the complex of GRK2, Gαq and Gβγ polypeptides. Position of a GTP molecule in Gαq active centre is indicated. B. Computer model of the electrostatic interaction between a putative phosphorylated GRK2-Ser121 residue and Arg214 of Gαq. C: Surface models for GRK2 protein in the vicinity of Ser121 residue. Left: Unphosphorylated Ser121; centre: model for the putative phosphorylated state of Ser121. Right: complementarity between the positively Arg214 and negative pSer121charged residues patched in both protein surfaces, probably implicated in the stabilization of the complex. D. Root mean square deviation (RMSD) plots of the protein domains implicated in the GRK2-Gαq interaction in presence (green) or absence (red) of phosphorylated Ser121 during a simulation of molecular dynamics. Plots are presented solely to illustrate the putative stabilization of the complex after Ser121 phosphorylation. Figure plots were generated using PyMOL Molecular Graphics System, Schrödinger, LLC.