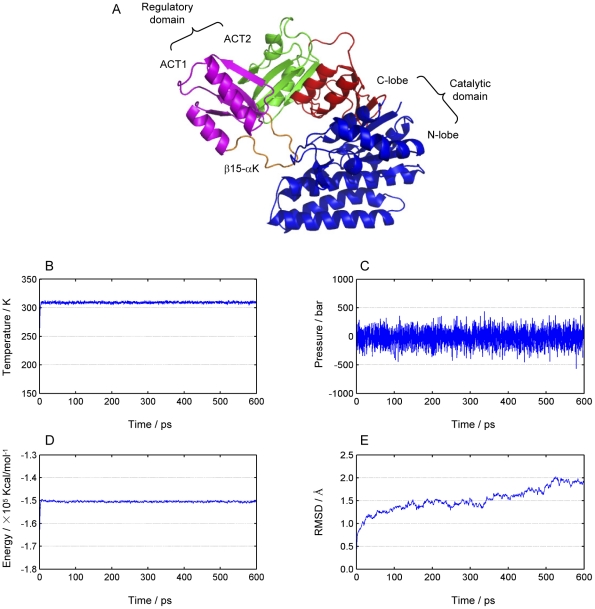
Figure 1. Structural regions of E. coli aspartokinase III and system parameters monitored during the molecular dynamics simulation.
(A) The structure is organized into a C-terminal regulatory region and an N-terminal catalytic region. The C-terminal regulatory region consists of two ACT domains, in which the second ACT domain (colored in green) is inserted within the first (colored in purple) via connections in two β-strands. ACT1 exhibits the fold of a typical ACT domain with an extended 14-residue loop between β15 and αK (colored in orange). The catalytic region exhibits a typical amino acid kinase family fold which can be further divided into the N-terminal lobe (N-lobe, colored in blue) and the C-terminal lobe (C-lobe, colored in red). (B) Temperature of the system. (C) Pressure of the system. (D) Total energy of the system. (E) Backbone root-mean-square deviations (RMSD) calculated from the trajectory using the first configuration as the reference and all coordinate frames from the trajectories were first superimposed on the initial conformation.