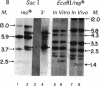
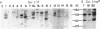Abstract
There is considerable evidence that infection by avian lymphoid leukosis viruses can led to tumor development in the target organ of the host. The mechanism by which virus-induced oncogenic transformation occurs, however, is not clearly understood. As a first step toward deciphering this process, we have characterized the proviruses of the lymphoid leukosis viruses in DNAs extracted from the leukotic and metastatic tumors by using restriction enzyme digestion and filter hybridization analysis with radioactive probes specific for the infecting genome. Our results indicate (i) that lymphoid leukosis tumors are clonal in origin; (ii) that there are multiple sites in the cellular genome of the target tissue where the virus DNA can integrate and that, in the majority of the tumors, at least one such site of each tumor is adjacent to a cellular sequence related to the oncogene of MC-29 virus; and (iii) that deletions and other structural alterations in the proviral DNA may facilitate tumorigenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffin J. M., Champion M., Chabot F. Nucleotide sequence relationships between the genomes of an endogenous and an exogenous avian tumor virus. J Virol. 1978 Dec;28(3):972–991. doi: 10.1128/jvi.28.3.972-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Payne L. N., Dent P. B., Burmester B. R., Good R. A. Pathogenesis of avian lymphoid leukosis. I. Histogenesis. J Natl Cancer Inst. 1968 Aug;41(2):373–378. [PubMed] [Google Scholar]
- Friedrich R., Kung H. J., Baker B., Varmus H. E., Goodman H. M., Bishop J. M. Characterization of DNA complementary to nucleotide sequences at the 5'-terminus of the avian sarcoma virus genome. Virology. 1977 Jun 1;79(1):198–215. doi: 10.1016/0042-6822(77)90345-2. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Vogt P. K., Nicolson M. O., McAllister R. M. Electron microscope studies of tumor virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):827–834. doi: 10.1101/sqb.1974.039.01.096. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Das S., Macdonnell D., McMillin-Helsel C. Organization of shared and unshared sequences in the genomes of chicken endogenous and sarcoma viruses. Cell. 1977 Jun;11(2):321–329. doi: 10.1016/0092-8674(77)90048-4. [DOI] [PubMed] [Google Scholar]
- Neiman P., Payne L. N., Weiss R. A. Viral DNA in bursal lymphomas induced by avian leukosis viruses. J Virol. 1980 Apr;34(1):178–186. doi: 10.1128/jvi.34.1.178-186.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Hughes S. H., Varmus H. E., Bishop J. M. Structure of viral DNA and RNA in mammalian cells infected with avian sarcoma virus. J Mol Biol. 1980 Nov 15;143(4):363–393. doi: 10.1016/0022-2836(80)90218-1. [DOI] [PubMed] [Google Scholar]
- Robinson H. L. Inheritance and expression of chicken genes that are related to avian leukosis sarcoma virus genes. Curr Top Microbiol Immunol. 1978;83:1–36. doi: 10.1007/978-3-642-67087-9_1. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Swanson C. A., Hruska J. F., Crittenden L. B. Production of unique C-type viruses by chicken cells grown in bromodeoxyuridine. Virology. 1976 Jan;69(1):63–74. doi: 10.1016/0042-6822(76)90194-x. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Sheiness D. K., Hughes S. H., Varmus H. E., Stubblefield E., Bishop J. M. The vertebrate homolog of the putative transforming gene of avian myelocytomatosis virus: characteristics of the DNA locus and its RNA transcript. Virology. 1980 Sep;105(2):415–424. doi: 10.1016/0042-6822(80)90042-2. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Tereba A., Astrin S. M. Chromosomal localization of ev-1, a frequently occurring endogenous retrovirus locus in white Leghorn chickens, by in situ hybridization. J Virol. 1980 Sep;35(3):888–894. doi: 10.1128/jvi.35.3.888-894.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Recombinants between endogenous and exogenous avian tumor viruses: role of the C region and other portions of the genome in the control of replication and transformation. J Virol. 1980 Jan;33(1):238–249. doi: 10.1128/jvi.33.1.238-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Witter R. L., Crittenden L. B. Lymphomas resembling lymphoid leukosis in chickens inoculated with reticuloendotheliosis virus. Int J Cancer. 1979 May 15;23(5):673–678. doi: 10.1002/ijc.2910230513. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Jay G., Pastan I. Unusual features in the nucleotide sequence of a cDNA clone derived from the common region of avian sarcoma virus messenger RNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):176–180. doi: 10.1073/pnas.77.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., de Crombrugghe B., Pastan I. Identification of a functional promoter in the long terminal repeat of Rous sarcoma virus. Cell. 1980 Dec;22(3):787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]