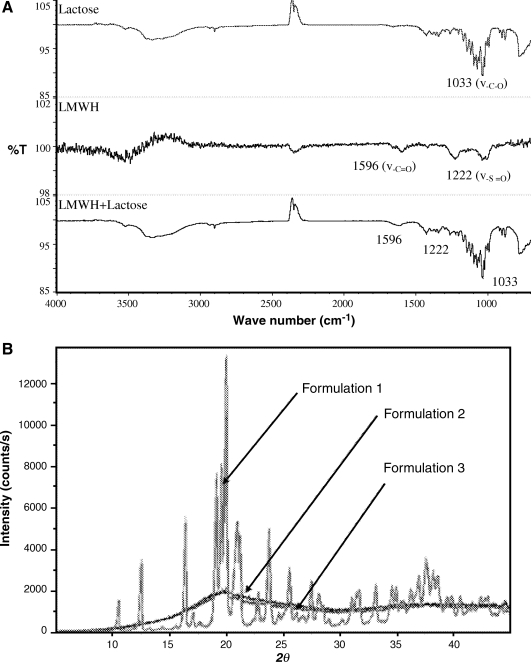
FIG. 1.
(A) FTIR analysis of a dry powder of enoxaparin solution, untreated lactose, and a lyophilized enoxaparin plus lactose (1:1 w/w) formulation; (B) X-ray diffractogram of a dry powder formulation of LMWH containing different concentrations of lactose: Formulation 1—LMWH in 5% lactose; Formulation 2—LMWH in 10% lactose; Formulation 3—LMWH in 15% lactose.