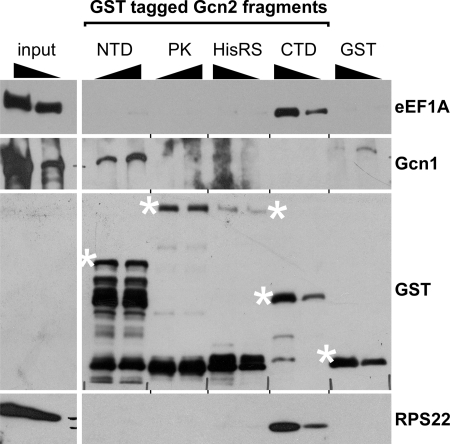
FIGURE 3.
Gcn2-CTD is sufficient for forming a complex with eEF1A. 4 and 2 μg of GST-tagged Gcn2 fragments encompassing the Gcn2 N terminus (NTD, amino acids 1–272, plasmid pB131), the protein kinase domain (PK, 568–998, pHQ551), the HisRS-like domain (HisRS, 970–1497, pHQ530), the Gcn2 C terminus (CTD, 1498–1659, pHQ531), or GST alone (pGEX-5x-1), expressed in E. coli, were incubated with glutathione-linked beads and purified. The immobilized fragments were then incubated with whole cell extract generated from the exponentially grown gcn2Δ strain H2557. Unbound proteins were removed, and the precipitate was subjected to SDS-PAGE and immunoblotting using antibodies against GST, Gcn1, eEF1A, and the ribosomal protein RPS22. 20 μg (10%) and 10 μg (5%) of the gcn2Δ WCE was loaded (input). The full-length GST fusion proteins are indicated with asterisks.