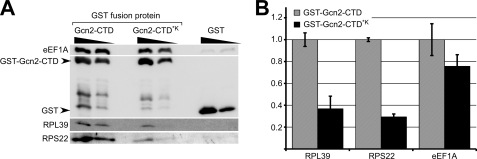
FIGURE 4.
Lys substitutions in the Gcn2-CTD affect ribosome co-precipitation more than eEF1A binding. A, various amounts (4 and 2 μg) of GST-Gcn2-CTD, of the same GST fusion protein but with K1552L/K1553I/K1556I substitutions (GST-Gcn2-CTD*K), or of GST alone as control, were subjected to co-precipitation assays and immunoblotting as described in Fig. 3. B, amount of proteins co-precipitated by the GST fusion proteins in A was quantified using the program ImageJ and determined relative to the precipitated amount of the respective GST fusion protein. These values were plotted relative to the co-precipitated values of GST-Gcn2-CTD. The standard errors are indicated as error bars. According to the t test the Lys substitutions significantly affected the Gcn2-CTD mediated co-precipitation of RPS22 (p value 0.002) and RPL39 (p value 0.033), whereas eEF1A co-precipitation was not significantly affected (p value 0.160).