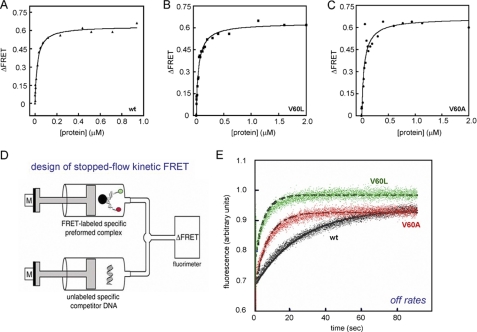
FIGURE 6.
FRET-based measurements of protein-DNA interactions. A–C, equilibrium binding studies of SRY and variants. D, schematic representation of stopped-flow experimental design (adapted from Ref. 34). The stopped-flow apparatus coupled to the fluorimeter allowed the measurement of FRET-based dissociation of SRY-DNA complex. One syringe contained a preformed protein-DNA complex containing a 15-bp DNA probe containing 5′-donor (fluorescein; green circle) on one strand and 5′-acceptor (TAMRA; red circle) on the other; the other syringe contained a 20-fold excess of unmodified DNA site. E, representative data and fitted solid lines at 15 °C showing time-dependent increase in donor fluorescence because of dissociation of FRET-labeled SRY-DNA complex: wild-type (wt)-complex (black), V60A complex (red), and V60L (green). Dissociation reactions were monitored for 90 s until equilibrium was reached. The dissociation rate constants (koff) were determined by fitting 5–7 individual traces to a single exponential equation and averaging the results.