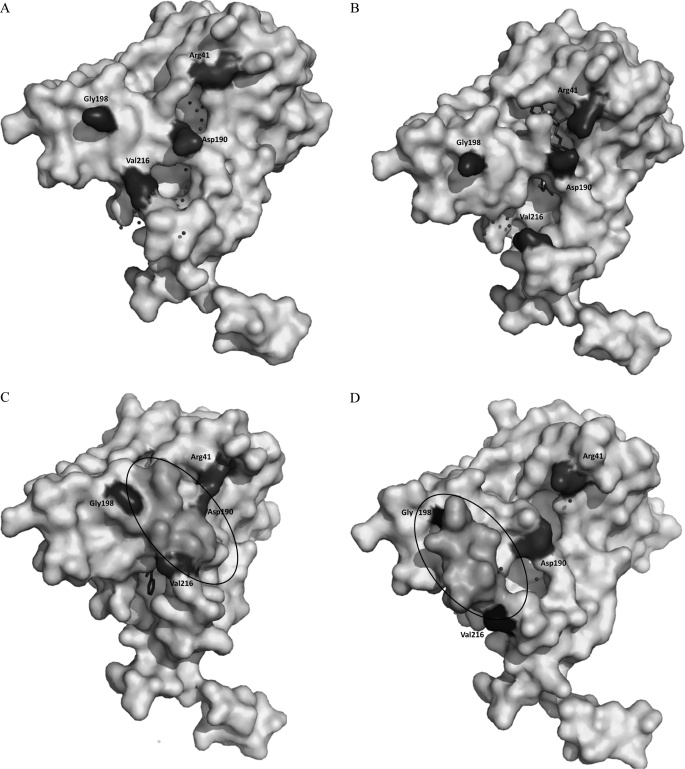
FIGURE 4.
The surface representation of BphBB-356 in (A) apo form, (B) binary form, and (C) ternary structures in the presence of 23-DB and the (D) intermediate state. Residues Asp190 and Arg41 are highlighted; in apo and binary structures they appear as separate residues, whereas in the ternary structure they appear to be joined due to the interaction between them. The circled region is part of substrate binding loop between Gly198 and Val216, which is not visible in apo and binary forms but is visible in the ternary and intermediate structures. Shift in the substrate binding loop in the ternary and intermediate regions could be seen. The spheres surrounding the coenzyme binding site and the active site show water molecules.