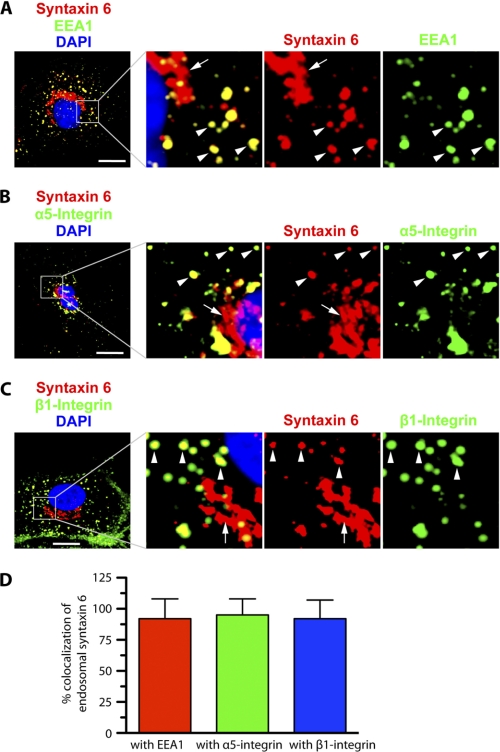
FIGURE 2.
Syntaxin 6, α5 integrin, and β1 integrin co-localize at EEA1-positive early endosomes in endothelial cells. HUVECs cultured in complete medium on fibronectin-coated surfaces were fixed, permeabilized, incubated with the indicated primary Abs, incubated with appropriate secondary Abs, and imaged by confocal fluorescence microscopy. A-C, representative confocal z-stacks, with enlarged insets highlighting colocalization between syntaxin 6 and EEA1, integrin α5, and integrin β1. In each case, arrowheads indicate colocalization between endosome-associated syntaxin 6 and each protein; arrows indicate lack of colocalization between Golgi-localized (perinuclear) syntaxin 6 and each protein. Confocal z-planes corresponding to the PM (dorsal- and ventral-most) are not included in B and C to avoid interference of signal from cell surface with signal from intracellular structures. D, signal overlap in images such as those were used to quantitate the extent to which syntaxin 6 was colocalized with each protein within EEs. Values represent mean ± S.D. (n = 50 cells for each condition, from 5 separate experiments; p ≤ 0.05). Scale bar represents 5 μm.