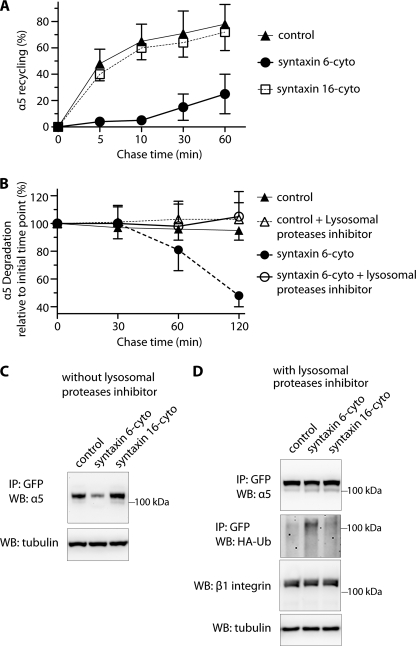
FIGURE 4.
Inhibition of syntaxin 6 function reduces endocytic recycling of α5 integrin and increases its ubiquitination and degradation in lysosomes. A, uninfected (control) and syntaxin 6-cyto- or syntaxin 16-cyto-infected HUVECs were surface biotinylated. Samples were then incubated at 16 °C for 30 min, to allow biotinylated α5β1 integrin to accumulate in EEs. Biotin remaining at the surface was removed by treatment with MesNa and quenching of MesNa with iodoacetamide (0-min time point); samples were further incubated at 37 °C for the indicated periods and, at each time point shown, subjected to a second MesNa treatment and then assessed for recycling of internalized integrin. After each time point, the cells were lysed and the amount of biotinylated integrin was determined by capture ELISA, using an Ab against α5 integrin. The fraction of internalized integrin recycled back to the PM is expressed as a percentage of surface-labeled protein originally internalized (from the 0-min chase time point). B, uninfected and syntaxin 6-cyto-infected cells were surface biotinylated and assessed for α5 integrin expression as in A, but were not subjected to a second treatment with MesNa. The fraction of internalized integrin remaining (i.e. PM-associated due to recycling + intracellular) after various chase periods is expressed as the percentage of surface-labeled internalized (0-min chase time point). Values in A and B are mean ± S.D. from four independent experiments; p ≤ 0.005 in A, p ≤ 0.05 in B. C and D, uninfected (Control) and syntaxin 6-cyto- or syntaxin 16-cyto-infected HUVECs after 12 h of infection were electroporated with (i) α5-integrin-GFP plasmid (in C) or (ii) a mixture of α5-GFP-integrin and HA-ubiquitin plasmids. After 12 h, the cells were cultured in the presence of 300 μm leupeptin for 12 h (in D). After 24 h of electroporation, cell lysates were prepared and incubated with anti-GFP Ab coupled to beads. Relative levels of α5-GFP-integrin in the cell lysates were determined by immunoprecipitating (IP) with anti-GFP Ab, and immunoblotting (WB) with anti-α5 integrin Ab. Ubiquitination was detected by immunoblotting against anti-HA Ab. Relative enrichment of β1 integrin and tubulin in cell lysates (10% input) was detected using Abs against β1 integrin and tubulin.