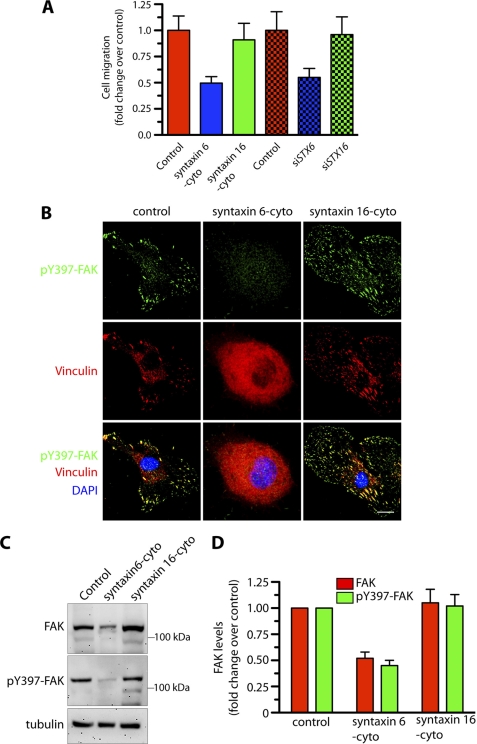
FIGURE 6.
Inhibition of syntaxin 6 function in endothelial cells blocks migration and recruitment of active FAK to focal adhesions. Uninfected (control), syntaxin 6-cyto-, syntaxin 16-cyto-, siSTX6-, or siSTX16-expressing HUVECs were grown on fibronectin-coated surfaces were serum starved for 2–3 h before being used for experiments. A, directional migration of HUVECs toward fibronectin in a Boyden chamber assay, with the medium in the lower well lacking serum. The number of migrating cells was normalized to that in uninfected controls. B, cells were stimulated with serum-containing medium for 10 min and then co-stained with Abs against phospho-Tyr397-FAK and vinculin to visualize focal adhesion sites. C, cells treated as in B were used to prepare lysates. Proteins were subjected to SDS-PAGE and immunoblotting with anti-FAK and anti-phospho-Tyr397-FAK Abs. Relative levels of tubulin in cell lysate confirms that protein loading in each well was equal. D, quantification of FAK and pFAK band densities; values represent ratios of pFAK to total FAK after normalization to the arbitrary value of 100 for uninfected HUVECs (control). Values represent mean ± S.D. (n = 3; p ≤ 0.005). Scale bars represent 5 μm.