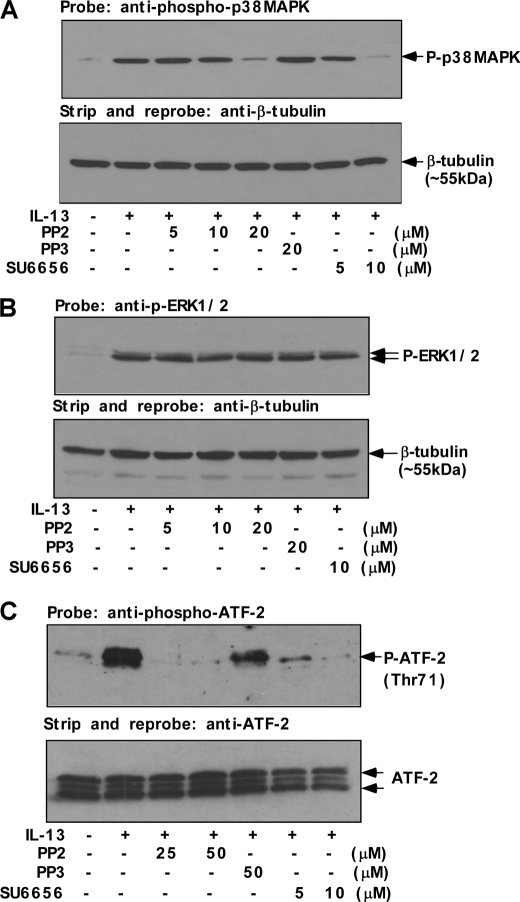
FIGURE 3.
Src kinase is the upstream regulator of p38 MAPK activity. Human monocytes (5 × 106/group) (A–C) were either directly treated with IL-13 for 15 min (A and C) and 1 h (B) or pretreated with Src kinase inhibitors SU6656 and PP2 or its inactive structural analog PP3 for 30 min at various indicated doses followed by IL-13 treatment for 15 min (A and C) and 1 h (B). A, postnuclear extracts (50 μg/lane) were resolved by 8% SDS-PAGE and immunoblotted with anti-phospho-p38 MAPK (Thr-180/Tyr-182) antibody (upper panel). The blot was subsequently stripped and reprobed with β-tubulin antibody to assess equal loading (lower panel). B, the cells were lysed, and 50 μg of the cell extracts (from each sample group) were separated by SDS-PAGE and immunoblotted with anti-phospho-ERK1/2 antibody (upper panel of B). The same blot was stripped and reprobed with β-tubulin antibody (lower panel) as a loading control. C, p38 MAPK enzymatic activity was measured in postnuclear extracts by selective IP using immobilized phospho-p38 MAPK (Thr-180/Tyr-182) antibody followed by incubation of IP pellets in kinase buffer containing ATF-2 fusion protein and cold ATP. The immunoprecipitates were resolved by SDS-PAGE and ATF-2 phosphorylation was detected using anti-phospho-ATF-2 (Thr-71) antibody by Western blotting. The same blot was stripped and reprobed with ATF-2 antibody as a loading control (lower panel).