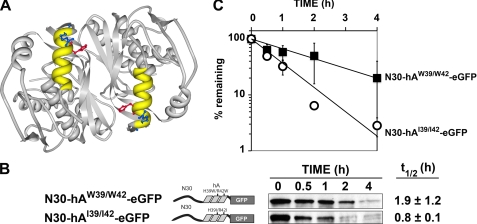
FIGURE 2.
Impact of substitutions at residues 39 and 42 within the hA helix. A, three-dimensional structure of the hTS dimer, generated using Protein Workshop (31), is depicted. The two hA helices, shown in yellow, occur in an antiparallel configuration relative to one another; the side chains of His-39 and Arg-42, shown in red and blue, respectively, point into the cleft created by the interface of the two subunits. B, plasmids expressing fusion protein N30-hAW39/W42-eGFP or N30-hAI39/I42-eGFP were stably transfected into cell line RJK88.13 and treated with CHX. Decay of eGFP concentrations over the indicated times was monitored by Western blotting (see “Experimental Procedures” for details). Schematic diagrams of the constructs are shown, along with representative blots. The half-lives (mean ± S.D.) are shown to the far right. C, first order decay plots of the data in B, from which half-lives were determined. Error bars, S.D.