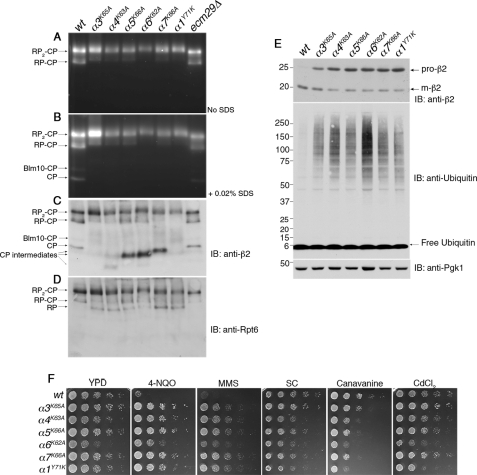
FIGURE 1.
Pocket lysine substitutions in CP α subunits result in CP assembly defects. A and B, whole cell extracts (70 μg) from indicated strains were resolved on 3.5% native gels and subjected to in-gel peptidase assays with fluorogenic substrate LLVY-AMC. The addition of SDS activates latent CP. supplemental Table S1 lists the yeast strains used in each figure henceforth. C and D, following the activity assays in A and B, the native gels were immunoblotted (IB) for a subunit of the CP (β2) and a subunit of the RP (Rpt6). E, whole cell extracts from A were subjected to 4–12% SDS-PAGE and immunoblotting. Pro-β2 is an immature, propeptide-containing form of β2, and m-β2 is the mature subunit. Pgk1 is a loading control. F, phenotypic analysis of the pocket lysine mutants. 5-fold serial dilutions of yeast strains were spotted on the indicated plates and incubated for 2–3 days at 30 °C. SC is synthetic complete medium. For testing sensitivity to canavanine (an arginine analog), arginine was omitted from the SC medium. The concentrations of the compounds tested were 0.4 μg/ml 4-NQO, 0.035% MMS, 1.5 μg/ml canavanine, and 30 μm CdCl2.