Table 2.
Method Ca: optimal conditions (% isolated yields)
| Entry | Carbonyl | Fulvene | (%) |
|---|---|---|---|
| a | 98 | ||
| b | 97 | ||
| c | 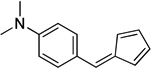 |
98 | |
| eb | 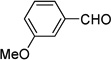 |
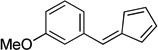 |
95 |
| f | 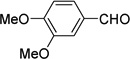 |
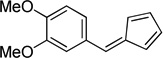 |
98 |
| gb | 97 | ||
| hb | 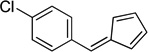 |
86 | |
| kb | 77 | ||
| le | 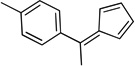 |
11 | |
| nb | 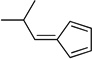 |
90 | |
| ob | CH3CHO | 90 | |
| pb | 85 | ||
| qb | 92 | ||
| rb | 95 | ||
| sb,c | 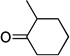 |
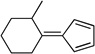 |
44 |
| vb,d | 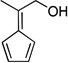 |
60 | |
| w | 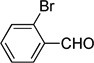 |
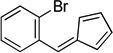 |
25 |
| x | 30 |
Method C: same as B, no molecular sieves, 5 mL MeOH-H2O (4:1).
To a solution of 1o (20 mmol) and CP (10.0 mmol), in MeOH-H2O (10 mL 7/3) pyrrolidine (10 mol%,) was added, and the reaction mixture stirred for 2.5 h at 15 °C. The mixture was poured into an ice-cold mixture of brine solution (10 mL) containing AcOH (1 mmol). The upper oil phase was separated and dried over molecular sieves.
To a solution of 2s (5 mmol) and CP (10 mmol) in 5 mL of methanol-water (4:1), pyrrolidine (10 mmol, 2 equiv) was added, and the mixture stirred at room temperature overnight. The mixture was subjected to flash chromatography on silica gel to give 3s in 44% yield.
Due to anticipated greater water solubility of product, extractive workup was used.
3equiv of pyrrolidine was used: reaction time 24 h.
