Table 1.
Ke values for ring A and N6 analogs at 5-HT2A and α1A receptors
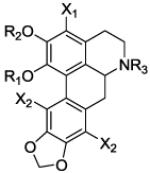 |
|||||||
|---|---|---|---|---|---|---|---|
| Compd | R1 | R2 | R3 | X1 | X2 | Ke ± SEM (nM)a |
|
| 5-HT2A | α 1A | ||||||
| 6a | n-Hex | Me | Me | H | H | 71±19 | >10,000 |
| 6b | Isobu | Me | Me | H | H | 367±82 | >10,000 |
| 6c | Isopen | Me | Me | H | H | NDb | >10,000 |
| 6d | 2-EthylBu | Me | Me | H | H | 806±152 | >10,000 |
| 6e | CyclobutylMe | Me | Me | H | H | NDb | >10,000 |
| 6f | CyclopentylMe | Me | Me | H | H | NDb | >10,000 |
| 6g | CyclohexylMe | Me | Me | H | H | 1722±258 | >10,000 |
| 6h | Allyl | Me | Me | H | H | 70±15 | >10,000 |
| 6i | HydroxyPr | Me | Me | H | H | 708±316 | >10,000 |
| 14a | Me | Et | Me | H | H | 378±92 | 52±13 |
| 14b | Me | n-Pr | Me | H | H | 389±93 | 133±45 |
| 14c | Me | n-Bu | Me | H | H | 943±283 | 234±52 |
| 14d | Me | n-Pen | Me | H | H | >10,000 | 449±169 |
| 14e | Me | CyclopropylMe | Me | H | H | 484±123 | 195±86 |
| 14f | Me | Benzyl | Me | H | H | 154±76 | 1917±226 |
| 15 | Me | Me | Me | Br | H | 53±14 | >10,000 |
| 16 | Me | Me | Me | Br | Br | 43±11 | >10,000 |
| 18a | Me | Me | Et | H | H | >10,000 | 26±6 |
| 18b | Me | Me | n-Pr | H | H | >10,000 | 38±3 |
| 18c | Me | Me | n-Bu | H | H | >10,000 | 210±50 |
| 18d | Me | Me | n-Pen | H | H | >10,000 | 720±204 |
| 18e | Me | Me | CyclopropylMe | H | H | >10,000 | 319±60 |
| 2 | Me | Me | Me | H | H | 850±6c | 36±7 |
| prazosin | 1.1 ± 0.4 | ||||||
| ketanserin | 32c,d | ||||||
Values represent mean ± SEM for three independent experiments.
ND = Ke not determined (compounds were weak agonists).
Data from reference 25
IC50 determined in the presence of 5-HT EC80
