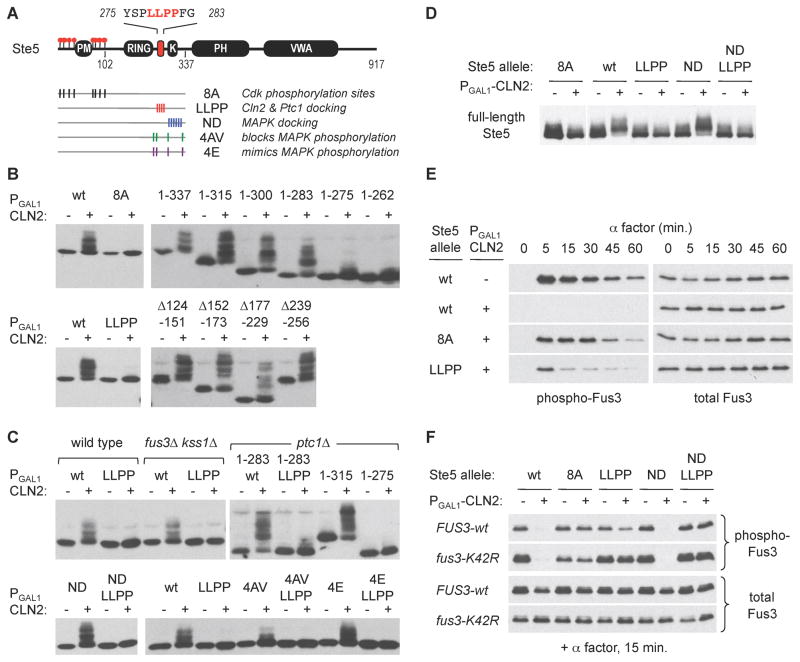
Figure 2. A distal docking motif promotes Cln2-Cdc28 phosphorylation of Ste5.
(A) Locations of key Ste5 features and mutations. Residues 275–283 contain the putative Cln2 docking motif required for efficient phosphorylation of the N-terminal Cdk sites. Mutations used in later panels are indicated; see Figure S1A for details
(B) Phosphorylation of the Ste5 N-terminus (Ste51–337) requires both the Cdk sites and a distal LLPP motif between residues 275 and 283. Phosphorylation was triggered by galactose-induced expression of a PGAL1-CLN2 construct (+) or a vector control (−)
(C) The role of the LLPP motif is independent of MAPKs (fus3Δ kss1Δ), the phosphatase Ptc1 (ptc1Δ), and the MAPK binding site (ND mutant). Results show the Ste51–337 fragment except as indicated otherwise. The fus3Δ kss1Δ strain (PPY1173) was tested in parallel with a congenic wild-type strain (PPY640). LLPP function also does not require the MAPK phosphorylation sites, but non-phosphorylatable mutations at these sites (4AV) mildly reduce the extent of Cln2-Cdc28 phosphorylation. Also see Figure S1B,C
(D) Cln2-driven phosphorylation of full-length Ste5 (V5-tagged) requires the LLPP motif. For the 8A lanes, a longer exposure (of the same blot) is shown to compensate for imperfect loading
(E) Mutation of the LLPP motif disrupts the ability of Cln2 to inhibit pheromone signaling. Pheromone-induced phosphorylation of Fus3 was monitored in ste5Δ fus3Δ kss1Δ strains (± PGAL1-CLN2) harboring STE5 variants and wild-type FUS3 on plasmids
(F) The LLPP motif mediates regulation by Cln2 in the absence of Fus3-Ste5 binding (Ste5 ND mutant) and Fus3 kinase activity (fus3-K42R mutant). Strains (as in panel D) harbored plasmids with the indicated forms of STE5 and FUS3.