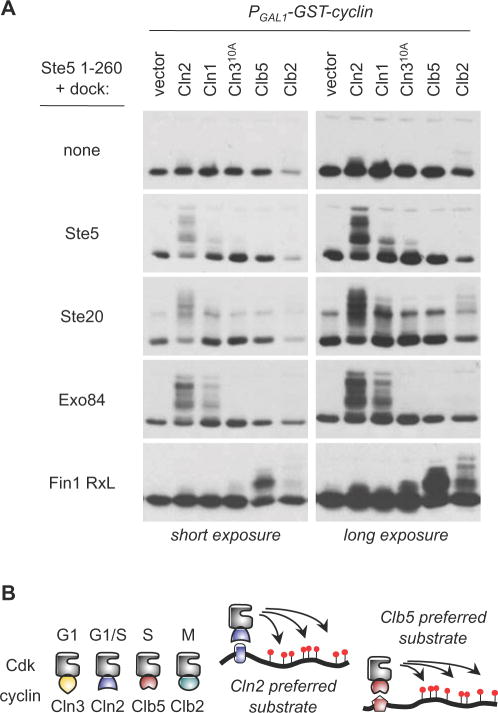
Figure 6. Docking sites drive cyclin-specific phosphorylation.
(A) Phosphorylation of a single substrate (Ste51–260) was monitored with and without the addition of the indicated docking sites, in cells expressing different PGAL1-induced cyclins. Docking sites from Ste5, Ste20, and Exo84 preferentially enhance phosphorylation by Cln1/2-Cdc28, whereas an RxL-containing fragment from Fin1 converts the substrate into one that is preferred by Clb5 and Clb2
(B) Schematic comparison of different cyclin-Cdk complexes, with a general model for how cyclin-specific docking interactions can selectively enhance substrate phosphorylation by individual forms of cyclin-Cdk.