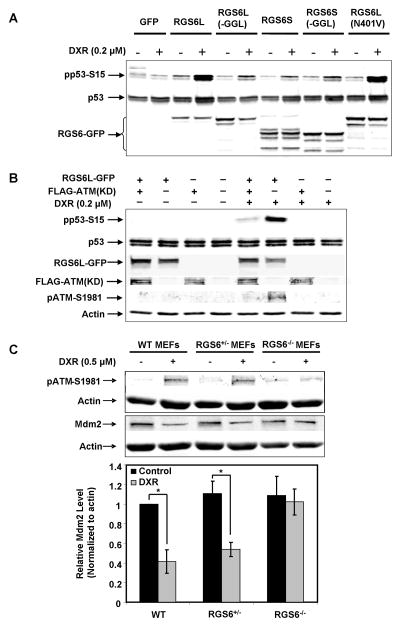
Figure 3. RGS6 promotes DXR-induced activation of p53 by mechanisms dependent upon ATM and independent of interactions with G proteins.
A. Comparison of the ability of RGS6 protein isoforms and a non-G protein-regulating mutant (RGS6LN401V) to promote activation of p53 by an ineffective dose of DXR. MCF-7 cells were transfected with GFP, GFP-tagged RGS6 isoforms or RGS6LN401V. Thirty-six h after transfection, cells were treated with vehicle or 0.2 μM DXR, a concentration insufficient to induce p53 phosphorylation without overexpression of RGS6L, for 6 h. A representative Western blotting result of three independent experiments is shown. B. RGS6L enhancement of DXR-induced p53 activation is blocked by a dominant negative ATM lacking its kinase activity (FLAG-ATM(KD)). MCF-7 cells were co-transfected with GFP or RGS6L-GFP and FLAG-ATM(KD) or pcDNA3. Thirty-six h after transfection, cells were treated with DXR as in A. A representative Western blot of three independent experiments is shown. C. Loss of RGS6 significantly blocks DXR-induced ATM activation as well as ATM-mediated phosphorylation of Mdm2. WT, RGS6+/−, and RGS6−/− MEFs were treated with 0.5μM DXR for 6 h. Upper panel representative results of three independent experiments. Lower panel quantification of Mdm2 immunoreactivity. Mdm2 level relative to actin in WT MEFs without DXR treatment was set as 1. Results represent means ± S.E. of three independent experiments (*p<0.005).