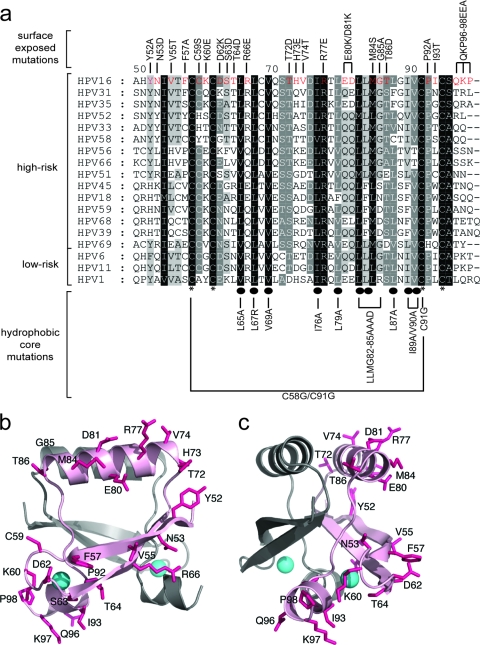
Fig. 1.
(a) Multiple sequence alignment of CR3 regions from representative supergroup A HPVs. Perfectly conserved residues are highlighted in black, with less conserved residues shaded in progressively lighter shades of gray, corresponding to the degree of conservation. The numbering scheme corresponds to HPV16 E7 CR3, starting with residue 49. Residues that are involved in zinc coordination are indicated with asterisks at the bottom of the alignment. Highly conserved hydrophobic core residues which are predicted to stabilize the dimer are labeled with ovals. The panel of mutations created for the purposes of the described studies is labeled at the top and bottom of the alignment. (b and c) Ribbon diagrams of the HPV16 model showing the positions of substitutions introduced into the protein. The model was produced using the PDB coordinates of HPV45 (2F8B) and a sequence alignment between the two proteins. The figures show the dimeric HPV16 model (a) and a view across a portion of the dimerization interface (b). The surface-exposed side chains where substitutions were made are shown as sticks (pink), and the bound zinc atoms are indicated as spheres (cyan). The two protomers are shaded differently, and substitutions are shown on only one protomer for clarity.