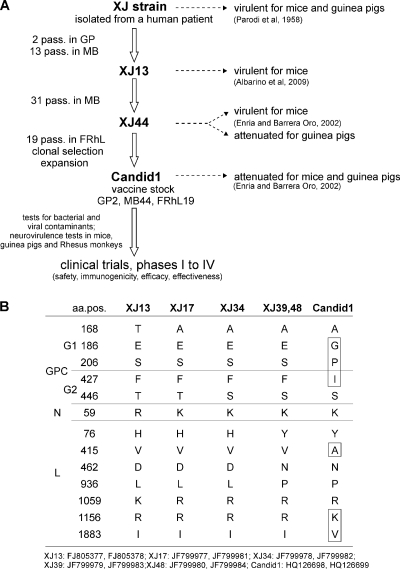
Fig. 1.
(A) Development of the Candid1 vaccine. This live-attenuated vaccine virus was originated from human clinical material and was passaged twice in guinea pigs (GP2) and 44 times in mouse (MB44), followed by clonal selection and stock amplification through 19 passages in fetal rhesus monkey lung cells (FRhL19). Virulence in mice and guinea pigs is indicated on the right side of the figure. (B) Sequence comparisons of Candid1 and its parental strains. For XJ-derived viruses (XJ13, XJ17, XJ34, XJ39, and XJ48), total RNA was purified from mouse brain lysates and used for reverse transcriptase PCR (RT-PCR) amplifications and sequencing. For Candid1, total RNA extracted from an original vial of the vaccine was used as input material. Amino acid positions (aa.pos.) correspond to each individual viral protein: GPC, N, and L. No mutations were found in Z or in nontranslated regions. XJ48 was derived from the Candid1 parental strain XJ44 and had a sequence identical to that of XJ39. GenBank accession numbers are listed for each strain.