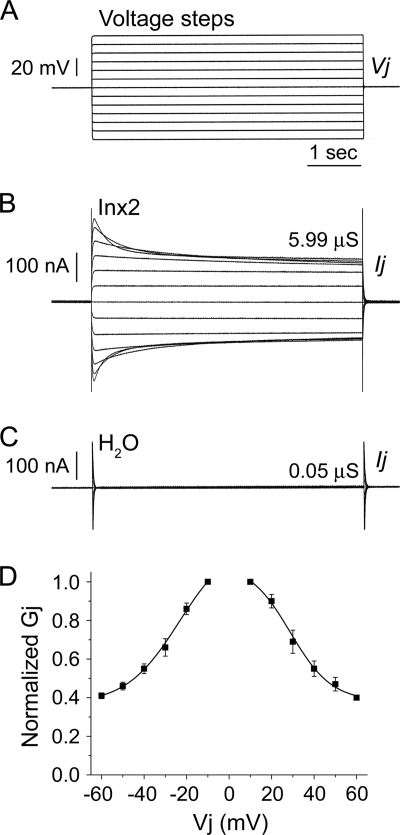
Fig. 1.
Spodoptera frugiperda Inx2 forms functional, voltage-dependent gap junction channels in vitro. Xenopus laevis oocytes were microinjected with 2 to 5 ng Sf-inx2 mRNA or water as a negative control. Following removal of the vitelline membranes, cells were paired and potential coupling was assessed 24 to 48 h later using the dual voltage clamp technique. Each cell of the pair was initially clamped to a holding potential (Vh) of −80 mV. Sequential depolarizing and hyperpolarizing voltage steps (A) were then applied to one cell of the pair (V1) while the second cell was maintained at Vh, generating a transjunctional potential difference, Vj (V1 − Vh, mV). The current Ij GAY required to maintain cell 2 at Vh was recorded and the transjunctional conductance, Gj (μS), calculated as Ij/Vj. (B) Representative trace for a cell pair expressing Sf-inx2 mRNA. The channels display sensitivity to transjunctional voltage, observed by the decrease in the currents with increasing depolarization and hyperpolarization. (C) No currents were recorded from water-injected controls, indicating that these cell pairs are not electrically coupled. (D) Gj/Vj plot showing the steady-state Gjs recorded in response to Vj steps applied to Inx2-expressing cell pairs. Gjs are normalized to their values at ±10 mV and are shown as mean ± SEM (n = 4 pairs). Data are fitted to a Boltzmann equation (parameters in text).