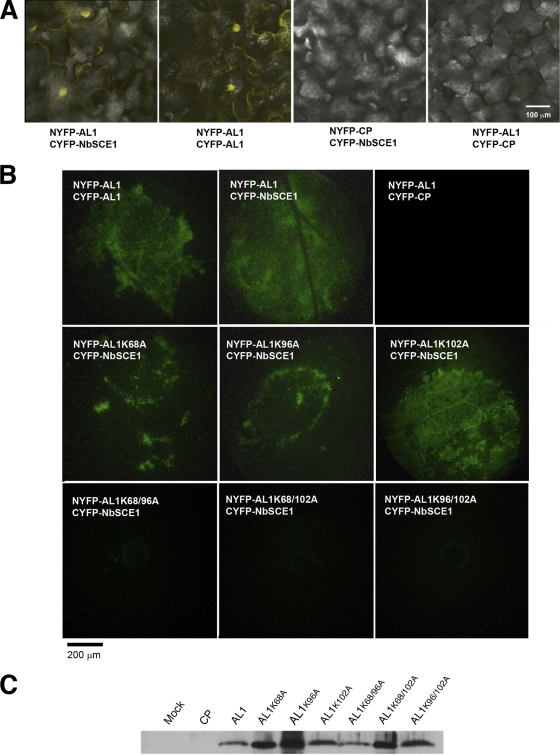
Fig. 3.
BiFC analyses showing in vivo oligomerization of AL1 and interaction with NbSCE1. N. benthamiana leaves were coagroinfiltrated with constructs fused to the C terminus (CYFP) or N terminus (NYFP) of YFP. Reconstituted YFP fluorescence was monitored 3 days after infiltration with a confocal microscope (A) or an epifluorescence binocular microscope with a Leica 10446364 filter for YFP emission (B). (A) Interaction of AL1 (wild type, AL1), NbSCE1, and CP from PNRV. (B) BiFC of AL1 (wild type and mutants) with NbSCE1. (C) Immunoblot analysis of protein extracts from leaves coagroinfiltrated with CYFP-NbSCE1 and wild-type or mutant AL1 or PNRV CP. Mock samples correspond to extracts from leaves agroinfiltrated with constructs expressing CYPF or NYFP. Fusion AL1 proteins were detected by using a polyclonal antibody against AL1. The molecular mass for the observed band is 58 kDa.