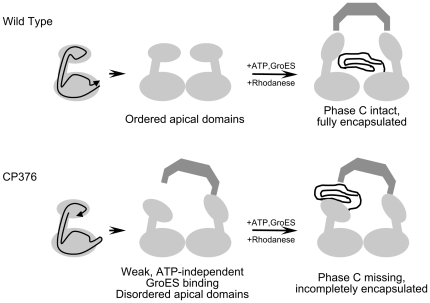
Figure 9. A summary of our findings regarding the CP376 GroEL mutant.
For simplicity, only one ring of the GroEL 14-mer is shown. In wild type GroEL (upper), the apical domains are arranged about the heptameric ring in an orderly fashion, and through Phase C, act in a coordinated manner to encapsulate and sequester folding intermediates of rhodanese. In CP376 (lower) however, this orderly orientation is disrupted, and both static and dynamic characteristics of encapsulation are affected. The dynamic aspects of apical domain disorder were observed by the disappearance of the Phase C kinetic transition in stopped-flow experiments, and the static consequences were reflected in an incomplete encapsulation of refolding rhodanese molecules that resulted in Proteinase K sensitivity of the football complex, as well as an ability to bind the cochaperonin GroES in the absence of ATP. Electron micrographs, CD spectra, and stopped-flow assays suggested that these results were caused by an increased flexibility of the apical domain as a functional unit, rather than by an unfolding of this domain caused by circular permutation (for example, the tryptophan fluorescence of CP376-RW continued to reflect various conformational changes of the GroEL subunit in a manner analogous to the wild type subunit).