Abstract
Previous studies have typically found that individuals with schizophrenia (SZ) report levels of emotional experience that are similar to controls (CN) when asked to view a single evocative stimulus and make an absolute judgment of stimulus “value.” However, value is rarely assigned in absolute terms in real-life situations, where one alternative or experience is often evaluated alongside others, and value judgments are made in relative terms. In the current study, we examined performance on a preference task that requires individuals to differentiate between the relative values of different stimuli. In this task, subjects were presented with many pairs of moderately positive stimuli and asked to indicate which stimulus they preferred in each pair. Resulting data indicated the rank order of preference across stimuli and the consistency of their transitive mapping (ie, if A > B and B > C, then A should be > C). Individuals with SZ (n = 38) were both less consistent in their rankings of stimuli and more likely to have larger magnitudes of discrepant responses than control subjects (n = 27). Furthermore, CN showed clear differentiation between different valence categories of stimuli (ie, highly positive > mildly positive > mildly negative > highly negative); while individuals with SZ showed the same general pattern of results but with less differentiation between the valence levels. These data suggest that individuals with SZ are impaired in developing or maintaining nuanced representations of the different attributes of a stimulus, thus making stimuli of similar general value easily confusable.
Keywords: schizophrenia, preference, reward, emotion, valence, anhedonia
Introduction
In the last decade, social cognition, emotion, reward processing, and motivation have emerged as central areas of investigation in the schizophrenia (SZ) research literature.1–4 This interest is likely the result of 2 factors. First, rapid advances in the basic affective neuroscience literature now provide a set of conceptual frameworks and experimental methods that offer traction on a number of long standing concerns in the SZ literature.5 Second, it is clear that more basic cognitive functions (eg, episodic memory, executive control) account for only a small portion of variance in functional outcome,6 suggesting that a fuller understanding of the deficits inherent to SZ requires a more comprehensive approach.
One of the central paradoxes in the SZ literature is the nature of emotional experience. Anhedonia has long been considered a central feature of the illness, in at least a substantial portion of patients.7 However, a large number of laboratory studies examining affective stimulus ratings in SZ have indicated that patients rate the valence and arousal of a variety of stimuli (eg, pictures, faces, words, drinks) similarly to controls (CN) (for a review, see Kring and Moran3). This was also recently confirmed by a meta-analysis of affective stimulus ratings in SZ, which indicated that patients do not display a hedonic deficit in the strictest sense (ie, rating positive stimuli as less pleasant).8 Thus, patient self-report of evoked emotional experience surprisingly provides no evidence to support the notion that individuals with SZ suffer from anhedonia.
Similarly, individuals with SZ are responsive to reward. There is evidence from implicit/procedural learning paradigms that patients are able to gradually learn from reinforcement, suggesting that rewards do in fact shape behavior9–15; however, this evidence is mixed.16–18 This type of gradual learning is thought to be largely mediated by the basal ganglia, bypassing deficits associated with hippocampal and prefrontal function that are associated with episodic and working memory systems.19
In contrast, classic studies using the Wisconsin Card Sorting Test,20 as well as more recent studies examining reversal learning and conditional associative learning paradigms, among others,21–25 suggest that patients have difficulty with rapidly updating working memory representations to guide response selection. There is also evidence that patients show alterations in decision making including steeper discounting of future rewards,26 failure to weigh the potential for losses in a gambling paradigm,9 and a reduced correlation between the amount of effort subjects made with their own reported emotional experience of stimuli.9 We recently suggested that it might be possible to account for this complex set of findings with the idea that patients have difficulty representing the value of multiple response alternatives or stimuli, a form of working memory for “value”1 of the sort encoded by orbitofrontal cortex (OFC).19,27
One limitation of the evidence to date is that impairments have primarily been observed in tasks that make a number of cognitive demands along with affective valuation and outcome processing. Thus, deficits in general working memory capacity could plausibly account for many of the deficits observed. In line with this explanation, we have found working memory (as measured through standard neuropsychological tests) to correlate with abnormalities in decision making in several studies.9,26 Alternatively, patients might process uncertainty differently than CN, and this could be implicated in many of the decision making and reinforcement learning experiments. That is, it is possible that impairments in “cold” cognitive processes might be largely responsible for impairments observed on many decision-making and reward processing tasks that were designed to target affective functions.
Another possibility is that patients display a deficit in assigning value in “relative” rather than “absolute” terms. In many of the studies examining affective ratings in SZ, subjects were required to evaluate a single stimulus at a time and make an absolute judgment regarding the affective value of that stimulus. However, value is rarely assigned to stimuli, actions, or events in absolute terms in real-life situations. Rather, the value of an item/action/event is typically determined when it is evaluated alongside other alternatives.28 For example, the affective value associated with the taste of a particular make of wine often increases or decreases when it is sampled alongside other wines. The OFC in particular has been shown to encode relative rather than absolute value.29 It is thus possible that the type of affective impairments that exist in SZ are more readily observable when patients are required to judge value in relative rather than absolute terms.
Preference judgment paradigms used in the affective neuroscience literature offer a novel means of examining relative value assignments, uncontaminated by cold cognitive impairments like working memory and problem solving. In these tasks, participants are typically presented with 2 stimuli and asked to select the stimulus that they prefer the most.30 There is no right or wrong answer on each trial, and no outcome is associated with the choice. Rather, participants simply indicate which of the 2 items they prefer over the other, for whatever reason. By presenting all the items paired with each other, it is possible to examine the consistency of choices made. More specifically, preference consistency is evaluated by examining the number of times each individual fails to maintain transitivity of preferences. For example, if a subject preferred A > B and B > C, they should prefer A > C. If they select C > A, this indicates an inconsistent preference judgment. A recent study by Fellows and Farrah31 demonstrated the role of the ventromedial prefrontal cortex (VMF: specified as the region encompassing both medial OFC and adjacent ventral medial PFC) in value-based preference judgments quite eloquently in a study on lesion patients. In this study, patients with VMF lesions, patients with frontal damage that spared the VMF (dorsal and/or lateral frontal, D/LF), and CN completed a 2-choice preference task. Results indicated that patients with VMF lesions displayed a greater number of inconsistent choices than other frontal lesion patients and CN, suggesting that the VMF is critically involved with representing the relative value of stimuli under conditions of certainty. These findings converge with studies demonstrating that OFC encodes relative rather than absolute value in both functional magnetic resonance imaging29 and monkey recordings32 and simulated in computational models of OFC to account for classical framing effects.19
If SZ patients have difficulty representing the relative value of stimuli in a precise fashion, we would expect them to demonstrate a higher level of response inconsistency and to make choices that deviated further from expectations based on prior choice history. In the current study, we applied a novel 2-choice preference judgment task31 to examine the possibility that individuals with SZ demonstrate abnormalities in representing the relative value of stimuli. It was hypothesized that individuals with SZ would make significantly more inconsistent choices on the preference task and that these inconsistent choices would be of significantly greater magnitude than those made by CN. We also predicted that both self-reported anhedonia and working memory would be significantly correlated with inconsistent choices, given that recalling past affective experiences and value-based decision making both rely on mental representations of affective value. This prediction is supported by neurocomputational models of the OFC, which suggest that this region maintains recent positive and negative valenced outcomes in an active working memory-like state, which is used to guide decision making via top-down projections.19 Based upon our previous behavioral data and computational model14 indicating that negative symptoms in SZ are associated with poor working memory for recent outcomes (thought to depend on intact OFC function) and hence poor value-based decision making, we predicted that the same mechanisms would lead to low choice consistency on the preference judgment task.
Materials and Methods
Participants
Participants included 38 individuals meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria for SZ or schizoaffective disorder (SZ, n = 37; schizoaffective, n = 1) (SZ) and 27 healthy CN. Individuals with SZ were recruited from the outpatient research program at the Maryland Psychiatric Research Center and evaluated during periods of clinical stability. Consensus diagnosis was established with a best-estimate approach based on psychiatric history and multiple interviews and subsequently confirmed using the Structured Clinical Interview for DSM-IV (SCID).33 All patients met DSM-IV lifetime diagnostic criteria for SZ or schizoaffective disorder and were prescribed antipsychotic medications at the time of testing. Patients were prescribed a variety of antipsychotic medications, either alone (Fluphenazine: n = 7; Clozapine: n = 6; Risperidone: n = 4; Haloperidol: n = 3; Olanzapine: n = 2; Ziprasidone: n = 1; Quetiapine: n = 1) or in combination with another antipsychotic (Clozapine + Risperidone: n = 1; Clozapine + Quetiapine: n = 2; Clozapine + Haloperidol: n = 1; Quetiapine + Olanzapine: n = 1; Quetiapine + Risperidone: n = 1; Quetiapine + Paliperidone: n = 1; Clozapine + Quetiapine + Haloperidol: n = 1), and were assessed after a minimum period of 4 weeks of stable treatment.
Control subjects were recruited through random-digit dialing and word of mouth among people who were recruited via random-digit dialing. All CN underwent a screening interview, including the SCID-IV, and did not meet lifetime criteria for a psychotic disorder or any current Axis I disorder. CN had no family history of SZ and no recent history of substance abuse (none within 6 months), which was confirmed by urine toxicology at the time of testing. Participants were also screened for lifetime neurological disorders and were free from significant neurological conditions.
SZ and CN did not significantly differ in age, F(1,64) = 0.33, P = .57, parental education, F(1,64) = 0.02, P = .90, gender, χ2 (1,64) = 0.29, P = .61, or ethnicity, χ2 (4,64) = 3.26, P = .52. Patients had lower Wechsler Abbreviated Scale of Intelligence (WASI) estimated full-scale intelligence quotients (IQs), F(1,64) = 12.83, P < .001, fewer years of total education, F(1,64) = 24.67, P < .001, and poorer scores on all composite scores from the MATRICS battery (all P’s < .05). Demographic and descriptive neuropsychological data are presented in table 1.
Table 1.
Demographic and Clinical Characteristics of Patients and Controls (CN)
| Schizophrenia, (n = 38) | CN, (n = 27) | |
| Age | 42.44 (10.34) | 41.00 (09.61) |
| Education | 12.43 (1.84) | 14.78 (1.88) |
| Parental education | 13.44 (3.30) | 13.35 (2.12) |
| WASI estimated full-scale intelligence quotients | 96.11 (14.06) | 109.42 (12.39) |
| % Male | 65.8% | 59.3% |
| Ethnicity | ||
| Caucasian | 55.3% | 48.1% |
| African American | 42.1% | 48.1% |
| Asian | 02.6% | 00.0% |
| Other | 00.0% | 03.7% |
| MATRICS battery | ||
| Processing speed | 35.23 (11.03) | 50.95 (11.11) |
| Attention/vigilance | 36.51 (13.18) | 50.80 (09.06) |
| Working memory | 37.42 (09.70) | 50.67 (11.63) |
| Verbal learning | 37.80 (08.92) | 44.19 (09.58) |
| Visual learning | 36.80 (13.21) | 46.95 (13.15) |
| Reasoning/problem solving | 40.20 (07.79) | 48.33 (07.57) |
| Social cognition | 35.91 (14.32) | 52.76 (10.37) |
| Overall | 29.00 (12.05) | 48.33 (11.13) |
Procedure
The preference task was administered as part of a larger battery of decision making, neuropsychological, self-report, and clinical symptom measures. Demographic and diagnostic data were collected prior to administration of computerized and paper and pencil neuropsychological tasks, and symptom interviews were conducted concurrently with the neurocognitive evaluations. Computerized and neuropsychological testing were conducted over a period of approximately 3–4 h, with breaks allowed as needed to diminish fatigue and maintain motivation. Most evaluations were conducted over 2 sessions, typically separated by 1 week. All participants received monetary compensation for participation.
Measures
CN and SZ participants completed the SCID-IV,33 as well as the Chapman Physical and Social Anhedonia Scales,34 which are self-report questionnaires designed to assess anhedonia. All participants also completed the full MATRICS battery35 to assess basic neuropsychological functioning. Symptom interviews were conducted for individuals with SZ, and patient’s therapists completed standard psychiatric rating scales, including the Brief Psychiatric Rating Scale (BPRS36) and Scale for the Assessment of Negative Symptoms (SANS37).
The preference judgment task was modeled after the task developed by Fellows and Farrah.31 In this task, 2 visual stimuli are presented concurrently on the computer screen, and subjects are required to select the stimulus that they prefer. Subjects were asked to make each preference judgment separately, and no mention was made that choices were to be made consistent. In instances where participants were clearly conflicted about 2 stimuli, the experimenter was instructed to say that there was no right or wrong answer and to encourage the participant to simply choose the picture they preferred most. When participants were presented with 2 negative stimuli, which the participant may not “like” per se, they were instructed to select the one that they “dislike less.”
A total of 6 conditions were presented. Four of these conditions consisted of color photographs representing distinct categories, including: fruit, landscapes, puppies, and vegetables. Examples of stimuli in each condition included: fruit (red apple, banana, kiwi), landscapes (rainforest, desert, mountains), puppies (German shepard, lab, shih tzu), and vegetables (broccoli, corn, cucumber). Subjects were presented with 66 trials in each condition, so that each of the 12 stimuli in a condition was paired with every other stimulus in that condition. The order of stimulus presentation was random within each condition, and the order of conditions was counterbalanced across subjects.
We also included 2 novel conditions to directly examine preference in relation to positively- and negatively-valenced images. These conditions, termed graded valence and same valence, consisted of 12 images per condition that were selected from the International Affective Picture System (IAPS38) stimulus set based upon published normative data. Stimuli in the graded valence condition consisted of 3 highly pleasant images (mean valence = 8.12; SD = 0.12), 3 mildly pleasant images (mean valence = 6.55; SD = 0.55), 3 mildly unpleasant images (mean valence = 3.56; SD = 0.54), and 3 highly unpleasant images (mean valence = 1.92; SD = 0.17), as determined by published normative IAPS ratings. In contrast, stimuli in the same valence condition were all moderately pleasant, consisting of images of people, animals, food, and everyday objects. These stimuli had a mean normative IAPS valence rating of 7.03 (0.09), with a range of 6.80–7.16 based upon the published norms. These images have been used in numerous studies on SZ, where it has generally been found that individuals with SZ and CN rate the affective valence of these stimuli nearly identically (for recent review and meta-analysis, see Kring and Moran3 and Cohen and Minor8). The inclusion of the graded valence and same valence conditions allowed for interesting comparisons, such as whether: (1) individuals with SZ make preference judgments in a normative fashion (ie, prefer highly positive > mildly positive > mildly negative > highly negative images) and (2) whether inconsistent preferences are limited to conditions that include only one semantic context (eg, all fruits, all puppies) or if they are characteristic of stimuli of similar valence that represent a range of semantic contexts (eg, hot air balloons, couple hugging, sports car). Data processing procedures were performed using a series of macros in Microsoft Excel, which were verified for accuracy by hand scoring.
Data Analysis
A series of data processing steps were taken in analyzing the preference data. First, we computed the initial rank order of each subject’s preferences for every condition by summing the total number of times each stimulus was chosen. Second, we took each subject’s initial rank order for each condition and calculated a “final rank order,” by applying multiple tie-breaking rules. Specific tie-breaking procedures were implemented for 2-way and 3-way or greater ties. For 2-way ties, the procedure entailed finding the “tie-breaking trial” where the tied items were judged by the subject and giving the preferred item in the tie-breaking trial the lower/better preference ranking (eg, if the Border Collie puppy was preferred over the Poodle puppy, then the Border Collie puppy has a rank of 4 and the Poodle puppy a rank of 5). For 3-way or greater ties, procedures consisted of finding all the trials where each of the tied items were compared to each other in order to determine whether one item won more tie-breaking trials than the others (eg, where Poodle is compared with Border Collie, Poodle is compared with Beagle, and Border Collie is compared with Beagle). The item winning the most tie-breaking trials was given the better/lower rank, and the item that lost more tie-breaking trials was given the worse/higher rank. In instances when all tied items were preferred an equal number of times on tie-breaking trials, all received the average rank of the 3 tied stimuli. Inconsistent choices were defined as choices that deviated from the final rank order of preferences. For example, if a subject chose the apple over orange and orange over banana, they were expected to choose apple over banana. If they instead chose banana over apple, this was considered an inconsistent choice. Fourth, the magnitude of inconsistency was determined for each inconsistent choice calculated by examining the number of rank-ordered items that fell between the selected less preferred rank-ordered item (ie, the inconsistent choice) and the more preferred rank-ordered item. For example, if the apple fell at 8 on the subject’s final rank-order list and the banana fell at number 2 and the apple was chosen over the banana, then this would result in an inconsistency magnitude of 6.
Primary dependent measures included the average of the total number of inconsistent choices made per condition, and the average magnitude of inconsistent choices made for each condition. MANOVAs and repeated measures ANOVAs were used to examine group differences, and Spearman correlations were calculated between primary dependent variables and symptom, neuropsychological, and self-report measures.
Results
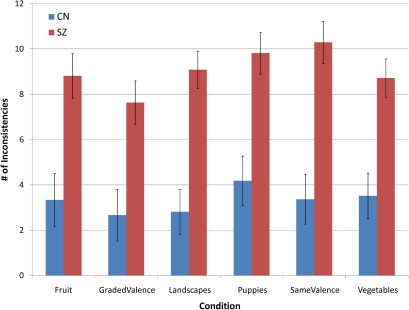
MANOVA indicated an overall difference between groups when we examined the total number of inconsistent choices made, F(6,58) = 5.18, P < .001 (η2 = .349). Significant differences were present between groups for all 6 conditions: fruit, F(1,64) = 12.80, P < .001, landscapes, F(1,64) = 23.81, P < .001, puppies, F(1,64) = 15.59, P < .001, vegetables, F(1,64) = 15.46, P < .001, graded valence, F(1,64) = 11.05, P < .001, and same valence, F(1,64) = 23.18, P < .001. Thus, results indicate that individuals with SZ made significantly more inconsistent choices than CN (see figure 1).
Fig. 1.
Average Count of Inconsistencies per Condition.
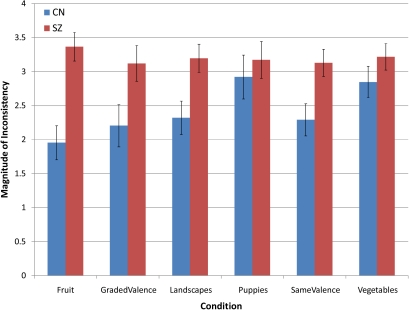
MANOVA also indicated that SZ subjects showed a higher average magnitude of inconsistent ratings than did CN subjects, F(6,58) = 4.08, P = .002 (η2 = .297). Significant differences were found on 4 out of 6 conditions, fruit, F(1,64) = 18.70, P < .001, landscapes, F(1,64) = 07.37, P = .009, graded valence, F(1,64) = 05.10, P = .027, same valence, F(1,64) = 07.37, P = .009. The analyses for puppies, F(1,64) = 0.35, P = .55, and vegetables, F(1,64) = 01.51, P = .22, were nonsignificant but also showed patients to make larger magnitude inconsistent choices. Thus, individuals with SZ show a general pattern of making larger magnitudes of inconsistent choices than CN (see figure 2) (After entering WASI full-scale IQ as a covariate, MANCOVA was significant for the total number of inconsistencies [F = 5.57, P < .001, η2 = .38] and magnitude of inconsistency [F = 3.83, P < .001, η2 = .30]. The group × emotion interaction also remained significant for the graded valence condition after controlling for IQ, F(3,63) = 5.95, P = .001 [η2 = .09]. Thus, our effects cannot be accounted for on the basis of group differences in IQ. We elected not to add these analyses to the main text due to problems related to the overmatching fallacy, whereby matching diagnostic groups on variables that are not independent of the illness causes variance directly attributable to the variable of interest to be removed due to overmatching on a variable, such as IQ. In studies examining patient groups, such as individuals with SZ, the overmatching fallacy may be particularly relevant due to the neurodevelopmental nature of the disorder).
Fig. 2.
Average Magnitude of Inconsistencies per Condition.
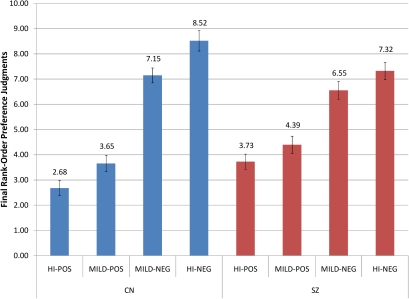
Fig. 3.
Mean Rank Order and SE for Stimulus Categories in the Graded Valence Condition. Lower values = more frequently preferred item (ie, rank of 1 = most frequently preferred item); higher values = less frequently preferred item (ie, rank of 12 = least frequently preferred item).
It is important to note that there were a similarly high number of inconsistent choices and large magnitude of inconsistent choices in the same valence condition as the fruit, landscape, puppies, and vegetables conditions. This suggests that failures of transitivity are not limited to conditions with a constrained semantic context but are also at hand when patients are presented with stimuli that are of similar valence but from a variety of semantic contexts.
We also examined whether SZ and CN made preference judgments in a normatively valenced fashion (ie, prefer highly positive > mildly positive > mildly negative > highly negative images) by examining preference assignments in the graded valence condition. Repeated measures ANOVA indicated a significant group (SZ vs CN) × condition (highly positive, mildly positive, mildly negative, highly negative) interaction, F(3,63) = 4.51, P = .01 (η2 = .07), as well as a significant main effect for Condition, F(3,63) = 79.69, P < .001 (η2 = .56); however, the between-subjects effect of group was nonsignificant, F(1,63) = 0.12, P = .73 (η2 = .00). The significant within-subjects effect indicates that the stimulus manipulation was successful and that stimuli were generally preferred in a normative fashion (ie, highly positive > mildly positive > mildly negative > highly negative). Paired-samples t-tests conducted separately for CN and SZ, indicated that CN preferred highly positive > mildly positive (t = −2.55, P = .02) and mildly negative > highly negative (t = −3.29, P = .003). In contrast, SZ patients did not prefer highly positive > mildly positive (t = −1.67, P = .16) nor did they prefer mildly negative > highly negative (t = −1.64, P = .11). Paired-samples t-tests also indicated that both CN and SZ preferred positive items significantly more than negative items (CN: t = −9.86, P < .001; SZ: t = −6.64, P < .001). Thus, although individuals with SZ show a normative pattern of preferring positive over negative items, they made less robust fine-grained distinctions within each valence category than CN (see figure 3).
Correlations between behavioral measures of frequency of inconsistency, magnitude of inconsistency, and rank-order preference on the graded valence condition (ie, the average preference position from 0 to 11 that items within each normatively defined valence category were ranked within that condition) with Chapman scale anhedonia and MATRICS working memory and global impairment are presented in table 2. Given our unique interest in the same valence and graded valence conditions, we presented correlations with these variables specifically; however, we also present correlations with the average inconsistency and magnitude across all 6 conditions for completeness. Results indicated that higher levels of physical anhedonia were associated with both a greater number of inconsistencies and a greater magnitude of inconsistency. Higher physical anhedonia levels were also associated with reduced rank-order preference for highly positive, mildly positive, and highly negative items in the graded valence condition, suggesting that more severe anhedonia is associated with a tendency to like positive stimuli less strongly and dislike negative stimuli less strongly. Working memory was significantly correlated with a greater magnitude of inconsistency but not greater numbers of inconsistent choices; however, general cognitive impairment was associated with both total number of inconsistencies and magnitude of inconsistency in the graded and same valence conditions. Furthermore, when we calculated separate positive and negative valence gradation scores (average rank order of high emotion – average rank order of mild emotion), these measures were significantly correlated with working memory (positive: r = 0.37, P < .03; negative: r = −0.39, P < .02), suggesting that poorer working memory performance is associated with less fine-grained distinctions between highly positive to mildly positive stimuli and mildly negative to highly negative stimuli. Positive and negative valence gradation scores were not significantly correlated with general cognition, suggesting that the ability to make fine-grained valence discriminations is specifically linked to working memory. Correlations between behavioral variables and the total and subscale global scores from the SANS, as well as BPRS positive, negative, and disorganized symptoms, were nonsignificant.
Table 2.
Correlations between Chapman Anhedonia Scales, Working Memory, and Behavioral Measures in Schizophrenia (SZ) Patients and Controls (CN)
| Chapman PA |
Chapman SA |
Working Memory |
General Cognition |
|||||
| SZ | CN | SZ | CN | SZ | CN | SZ | CN | |
| Inconsistencies | ||||||||
| Graded valence inconsistency | 0.41* | −0.10 | 0.19 | −0.13 | −0.17 | 0.08 | −0.32 | 0.26 |
| Same valence inconsistency | 0.31 | −0.08 | 0.15 | 0.08 | −0.20 | −0.06 | −0.43** | 0.04 |
| Total inconsistency | 0.32 | −0.35 | 0.20 | −0.11 | −0.16 | 0.05 | −0.40* | 0.13 |
| Magnitude | ||||||||
| Graded valence magnitude | 0.52** | 0.21 | 0.32 | 0.18 | −0.35* | −0.12 | −0.41* | −0.04 |
| Same valence magnitude | 0.24 | −0.06 | 0.17 | 0.09 | −0.37* | 0.02 | −0.46** | 0.16 |
| Total magnitude | 0.37* | 0.23 | 0.17 | 0.33 | −0.17 | 0.33 | −0.27 | 0.06 |
| Rank order preferences | ||||||||
| High positive | 0.34* | 0.22 | 0.14 | 0.14 | −0.21 | −0.36 | −0.02 | −0.23 |
| Mild positive | 0.34 | 0.03 | −0.16 | 0.03 | 0.22 | −0.18 | 0.20 | −0.24 |
| Mild negative | −0.20 | −0.31 | −0.07 | −0.47* | −0.33* | −0.16 | −0.28 | −0.22 |
| High negative | −0.35 | −0.02 | 0.08 | 0.13 | 0.26 | 0.51** | 0.08 | 0.48* |
Note: Chapman PA, Chapman Physical Anhedonia Scale; Chapman SA, Chapman Social Anhedonia Scale; working memory, MATRICS working memory domain T-score; general cognition, MATRICS overall cognition T-score; total inconsistency and total magnitude, average value across all 6 conditions; rank-order preferences, average rank-ordered value for items per valence range in the same valence condition, where lower values reflect greater preference.
Discussion
Previous studies have indicated that individuals with SZ rate the affective value of emotional stimuli similarly to CN3,8; however, these studies required value assignments to be made in absolute rather than relative terms. In the current study, we examined the novel possibility that SZ is associated with abnormalities in judging relative value when multiple affective stimuli are presented in a 2-choice preference task. Specifically, we tested the prediction that individuals with SZ would make a greater number of inconsistent choices and display a greater magnitude of inconsistency than CN. Results supported both of these hypotheses, and the number of inconsistent choices made by individuals with SZ was comparable with what has been seen in VMF lesion patients.31 At a behavioral level, these results reflect that SZ patients show less differentiation in their weighting of the subjective relative value of stimuli. These findings fit with a series of recent studies by our group that provide converging evidence that SZ patients display deficits in representing reward value (for a review, see Gold et al1). Our results are also relevant to the growing body of studies indicating that SZ patients evidence a form of affective ambivalence, whereby they report more negative reactions to positive stimuli and positive reactions to negative stimuli.8 It is possible that patients make such erratic responses because they process affective stimuli with a sense of uncertainty, which may arise due to difficulty representing stimulus value.
When interpreting the current results in relation to the broader SZ literature, it is important to note that different affective tasks appear to differentially activate closely related but different brain regions. In tasks where subjects are asked to make absolute affective evaluations, where they report their feelings in response to an affective stimulus, dorsal/rostral areas of the medial prefrontal cortex (mPFC) are activated, whereas more ventral areas of the mPFC are activated when subjects make a value judgment about the stimulus itself.39–41 In preference tasks where affective judgments are made in relative terms, such as the one employed in the current study, regions encompassing both medial OFC and adjacent ventral medial PFC are involved.31,42–47 We suspect that VMF dysfunction (referred to here as the region encompassing both medial OFC and adjacent ventral medial PFC) provides a neural basis for the diminished level of distinction made between stimuli and large number of inconsistencies given the roles of the VMF in making preference judgments,31,42–47 as well as consistent evidence of structural48–51 and functional52–54 abnormalities in these regions in SZ. However, functional neuroimaging studies using preference judgment tasks, along with tasks that require absolute judgments of feelings and stimulus ratings, are needed to confirm this interpretation and disentangle the brain regions associated with preserved and abnormal affective response in SZ.
The inclusion of our graded valence condition also allowed for interesting comparisons regarding the rank order of value judgments. In this condition, we presented subjects with highly positive, mildly positive, mildly negative, and highly negative IAPS images. When we examined the final rank-ordered preference, it was clear that the preferences of CN were much more in line with normed IAPS data than were the preferences of patients with SZ (ie, they preferred highly positive > mildly positive > mildly negative > highly negative). While individuals with SZ showed normal differentiation between positive and negative images broadly defined (ie, they preferred positive > negative), they neither did not prefer highly positive images to a significantly greater extent than mildly positive images nor did they prefer mildly negative images more than highly negative images. In this regard, findings are consistent with the notion that individuals with SZ show less fine-grained distinctions in their preferences for stimuli falling within positive and negative emotional categories.
Consistent with hypotheses, greater magnitude and number of inconsistent choices were significantly correlated with self-reported Chapman scale anhedonia in patients. However, it was curious that the magnitude of inconsistency and number of inconsistent choices were not significantly correlated with clinical symptoms, as rated by the BPRS or SANS. The reason for this dissociation is somewhat unclear; however, we expect that it might be due to differences inherent to the constructs tapped by these measures. Clinical interviews that measure negative symptoms, such as the SANS and BPRS, require patients to think back over their recent experiences during the past few weeks and indicate the extent to which they have performed certain behaviors. For example, the SANS rates the extent to which patients have lost interest in initiating pleasurable activities, whether they are able to form close relationships, and if they have engaged in behaviors aimed at obtaining reward. In contrast, the Chapman scales involve an unusual task demand, requiring a participant to call into mind some situation, which they may or may not have experienced very often or at all, and determine whether that experience describes how they might feel in that situation (eg, true or false—“I have usually found lovemaking to be intensely pleasurable”). In order to make this true–false judgment, participants are required to form some kind of mental representation of the value of the experiences that are being probed. Thus, it seems possible that clinical rating scales provide an index of reward-seeking behavior, while the self-report anhedonia scales tap into inner emotional experience, which may be more highly associated with the deficits in representing reward value observed on our preference task.
It was also interesting that the magnitude of inconsistency was significantly correlated with working memory. Correlations with working memory are consistent with the neurocomputational models suggesting that OFC maintains the relative magnitudes of positive and negative outcomes in working memory,19 such that OFC dysfunction in SZ compromises the ability to use working memory to represent and compare the relative value of different outcomes. These associations further suggest that working memory deficits may underlie the ability to couple affective value and behavior,9,55 although correlations with general cognition suggest that cognitive processes other than working memory may be at play as well. As we have previously noted,9 decision making and behavior are likely to be highly influenced by the extent to which individuals are able to generate and maintain stimulus representations in working memory. Without the ability to sustain mental representations of stimulus value, it is unlikely that affective value will carry enough salience to adequately influence behavior. Such representational failures may influence behavior in a profound way in paired-choice preference tasks by limiting the nuanced representation of the different attributes of a stimulus, making stimuli of similar general value easily confusable. These results are similar to those of Burbridge and Barch,56 who found evidence that working memory impairment moderates the relationship between anhedonia and self-reported emotional experience of positive stimuli. It is possible that dysfunction within PFC networks may explain the association between working memory and preference inconsistency, as these regions are implicated in performance on both tasks.31,42–47,57
In sum, results indicate that individuals with SZ show deficits in judging the relative value of affective stimuli, as indicated by a greater number and magnitude of inconsistent preference choices and reduced fine-grained distinctions of stimuli falling within the same affective category (ie, pleasant or unpleasant). When viewed in conjunction with previous work on preference judgment conducted in the cognitive/affective neuroscience literature,31,42–47,57 as well as previous work by our group and others on reward value and learning in SZ,9,55 findings are consistent with the idea that abnormalities in the subjective valuation of affective stimuli may result from difficulty forming mental representations and dysfunctional PFC networks.
Funding
US National Institutes of Mental Health grants R01 MH080066-01, 5P30MH068580-02, and 5T32MH067533-05.
Acknowledgments
We would like to thank Drs Lesley Fellows and Alex Henri-Bhargava for their expert consultation regarding task design and data processing, as well as for providing us with materials used in their experiments. We are also indebted to the subjects who participated in the study and staff at the Maryland Psychiatric Research Center who made the completion of the study possible. We are especially thankful to Sharon August, Leeka Hubzin, Lindsay Phebus, and Tatyana Matveeva, who conducted subject recruitment and testing.
References
- 1.Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green MF, Leitman DI. Social cognition in schizophrenia. Schizophr Bull. 2008;34:670–672. doi: 10.1093/schbul/sbn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van 't Wout M, van Rijn S, Jellema T, Kahn RS, Aleman A. Deficits in implicit attention to social signals in schizophrenia and high risk groups: behavioural evidence from a new illusion. PLoS One. 2009;4:e5581. doi: 10.1371/journal.pone.0005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 2008;64(1):48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 7.Kraepelin E. Dementia Praecox and Paraphrenia. New York, NY: Krieger; 1971. [Google Scholar]
- 8.Cohen AS, Minor KS. Emotional experience in schizophrenia patients revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 2008;64:62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keri S, Kelemen O, Szekeres G, et al. Schizophrenics know more than they can tell: probabilistic classification learning in schizophrenia. Psychol Med. 2000;30(1):149–155. doi: 10.1017/s0033291799001403. [DOI] [PubMed] [Google Scholar]
- 11.Morris SE, Heerey EA, Gold JM, Holroyd CB. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr Res. 2008;99:274–285. doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry W, Light GA, Davis H, Braff DL. Schizophrenia patients demonstrate a dissociation on declarative and non-declarative memory tests. Schizophr Res. 2000;46:167–174. doi: 10.1016/s0920-9964(99)00229-7. [DOI] [PubMed] [Google Scholar]
- 13.Stevens A, Schwarz J, Schwarz B, Ruf I, Kolter T, Czekalla J. Implicit and explicit learning in schizophrenics treated with olanzapine and with classic neuroleptics. Psychopharmacology. 2002;160:299–306. doi: 10.1007/s00213-001-0974-1. [DOI] [PubMed] [Google Scholar]
- 14.Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weickert TW, Terrazas A, Bigelow LB, et al. Habit and skill learning in schizophrenia: evidence of normal striatal processing with abnormal cortical input. Learn Mem (Cold Spring Harbor, NY) 2002;9:430–442. doi: 10.1101/lm.49102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen A, Siegmund A, Ohrmann P, et al. Reduced implicit and explicit sequence learning in first-episode schizophrenia. Neuropsychologia. 2008;46:186–195. doi: 10.1016/j.neuropsychologia.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Kumari V, Gray JA, Honey GD, et al. Procedural learning in schizophrenia: a functional magnetic resonance imaging investigation. Schizophr Res. 2002;57:97–107. doi: 10.1016/s0920-9964(01)00270-5. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz BL, Howard DV, Howard JH, Jr., Hovaguimian A, Deutsch SI. Implicit learning of visuospatial sequences in schizophrenia. Neuropsychology. 2003;17:517–533. doi: 10.1037/0894-4105.17.3.517. [DOI] [PubMed] [Google Scholar]
- 19.Frank MJ, Claus ED. Anatomy of a decision: striatoorbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg TE, Weinberger DR, Berman KF, Pliskin NH, Podd MH. Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study of teaching the Wisconsin Card Sorting Test. Arch Gen Psychiatry. 1987;44:1008–1014. doi: 10.1001/archpsyc.1987.01800230088014. [DOI] [PubMed] [Google Scholar]
- 21.Gold JM, Bish JA, Iannone VN, Hobart MP, Queern CA, Buchanan RW. Effects of contextual processing on visual conditional associative learning in schizophrenia. Biol Psychiatry. 2000;48:406–414. doi: 10.1016/s0006-3223(00)00930-6. [DOI] [PubMed] [Google Scholar]
- 22.Kemali D, Maj M, Galderisi S, Monteleone P, Mucci A. Conditional associative learning in drug-free schizophrenic patients. Neuropsychobiology. 1987;17:30–34. doi: 10.1159/000118337. [DOI] [PubMed] [Google Scholar]
- 23.Rushe TM, Woodruff PW, Murray RM, Morris RG. Episodic memory and learning in patients with chronic schizophrenia. Schizophr Res. 1999;35:85–96. doi: 10.1016/s0920-9964(98)00117-0. [DOI] [PubMed] [Google Scholar]
- 24.Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiler JA, Bellebaum C, Brüne M, Juckel G, Daum I. Impairment of probabilistic reward-based learning in schizophrenia. Neuropsychology. 2009;23:571–580. doi: 10.1037/a0016166. [DOI] [PubMed] [Google Scholar]
- 26.Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12:213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 28.Seymour B, McClure SM. Anchors, scales and the relative coding of value in the brain. Curr Opin Neurobiol. 2008;18:1–6. doi: 10.1016/j.conb.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Elliott R, Agnew Z, Deakin JF. Hedonic and informational functions of the human orbitofrontal cortex. Cereb Cortex. 2008;20(1):198–204. doi: 10.1093/cercor/bhp092. [DOI] [PubMed] [Google Scholar]
- 30.Paulus MP, Frank LR. Ventromedial prefrontal cortex activation is critical for preference judgments. Neuroreport. 2003;14:1311–1315. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- 31.Fellows LK, Farah MJ. The role of ventromedial prefrontal cortex in decision making: judgment under uncertainty or judgment per se? Cereb Cortex. 2007;17:2669–2674. doi: 10.1093/cercor/bhl176. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Patient Edition (SCID-I/P 2/2001 Revision) New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- 34.Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- 35.Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 37.Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City: University of Iowa Press; 1983. [Google Scholar]
- 38.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Digitized Photographs, Instruction Manual and Affective Ratings, Technical Report A-6. Gainesville, FL: University of Florida; 2001. [Google Scholar]
- 39.Wager TD, Barrett LF, Bliss-Moreau E, et al. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The Handbook of Emotions. 3rd ed. New York, NY: Guilford; 2008. pp. 249–271. [Google Scholar]
- 40.Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 41.Olsson A, Ochsner KN. The role of social cognition in emotion. Trends Cogn Sci. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage. 2002;15:983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]
- 43.Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunningham WA, Johnson MK, Gatenby JC, Gore JC, Banaji MR. Neural components of social evaluation. J Pers Soc Psychol. 2003;85:639–649. doi: 10.1037/0022-3514.85.4.639. [DOI] [PubMed] [Google Scholar]
- 45.McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Montague PR. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44:379–387. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 46.Johnson SC, Schmitz TW, Kawahara-Baccus TN, et al. The cerebral response during subjective choice with and without self-reference. J Cogn Neurosci. 2005;17:1897–1906. doi: 10.1162/089892905775008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volz KG, Schubotz RI, von Cramon DY. Decision-making and the frontal lobes. Curr Opin Neurol. 2006;19:401–406. doi: 10.1097/01.wco.0000236621.83872.71. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein JM, Goodman JM, Seidman LJ, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- 49.Crespo-Facorro B, Paradiso S, Andreasen NC, et al. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- 50.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 51.Davatzikos C, Shen D, Gur RC, et al. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 2005;62:1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- 52.Bertollo DN, Cowen MA, Levy AV. Hypometabolism in olfactory cortical projection areas of male patients with schizophrenia: an initial positron emission tomography study. Psychiatry Res. 1996;60:113–116. doi: 10.1016/0165-1781(96)02619-4. [DOI] [PubMed] [Google Scholar]
- 53.Carter CS, MacDonald AW, III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 54.Quintana J, Wong T, Ortiz-Portillo E, et al. Prefrontal-posterior parietal networks in schizophrenia: primary dysfunctions and secondary compensations. Biol Psychiatry. 2003;53:12–24. doi: 10.1016/s0006-3223(02)01435-x. [DOI] [PubMed] [Google Scholar]
- 55.Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- 56.Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol. 2007;116:30–42. doi: 10.1037/0021-843X.116.1.30. [DOI] [PubMed] [Google Scholar]
- 57.Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]