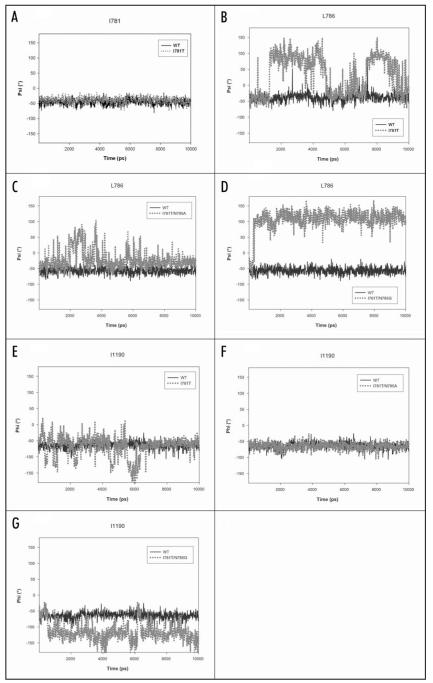
Figure 2.
Backbone angles of wild-type and mutant channels. (A) Backbone angle (ψ) of position 781 (domain II). Interestingly, there are no differences observable between wild-type (black line) and threonine mutant (gray, dotted line). This is also true for other mutant channels (data not shown). (B) Backbone angle (ψ) for amino acid L786 (domain II), which is located downwards of I781. Interestingly, this amino acid is relatively stable in wild-type but fluctuates drastically (up to 150°) in the I781T mutant channel. (C) Behaviour of L786 in the rescuing double mutant I781T/N785A channel. The backbone of L786 is more flexible in the I781T/N785A double mutant than wild-type but less flexible than in the I781T mutant and stabilizes gradually (B, above). (D) Backbone angle (ψ) of the non rescuing double mutant I781T/N785G channel. The backbone angle is more flexible than wild-type and adopts a conformation, usually seen in beta sheets. (E) Backbone angle of I1190 in IIIS6. This amino acid is involved in hydrophobic interactions to the neighboring IIS6 residue L786 in the open conformation of the wild-type channel (see also Fig. 4B). Because of the higher flexibility in this area introduced by single point mutations in position 781, interactions to IIS6 are lost (Fig. 4D) and the backbone conformation of I1190 fluctuates. (F) In the double mutant the interactions between L786 (IIS6) and I1190 (IIIS6) remain intact and the backbone of helix III remains stable. (G) Backbone angle of I1190 in the non rescuing double mutant I781T/N785G channel. In agreement with the higher flexibility at position L786 (domain II), I1190 looses hydrophobic contacts to L786 and shows considerable fluctuations.