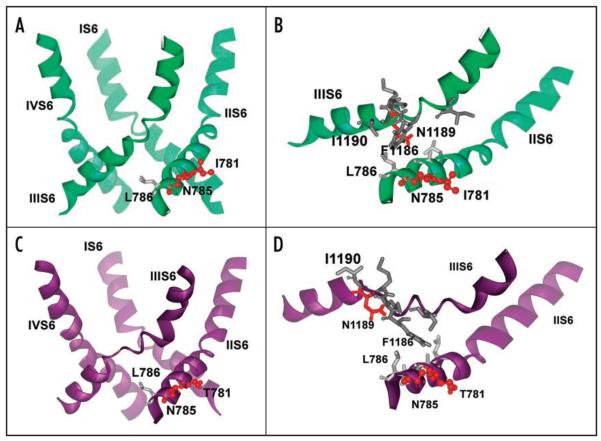
Figure 3.
Structural consequences of mutations on pore helix stability revealed by MD simulations. (A) Representative snapshot of the wild-type channel pore at the end of six ns simulation in ribbon presentation. No distortions in IIIS6 segment appear during our repeated simulations. (B) Details of amino acids involved in structural rearrangements upon mutation in position 781 to threonine. Polar amino acids are coloured red, while hydrophobic residues are gray. Position 781 is facing the opposite side and cannot directly interact with helix IIIS6. N785 is closer to the neighboring S6 segment and is connected to L786, which interacts with the highly conserved I1190 from helix IIIS6. The distance between L786 and I1190 is less than 4 Å. (C) Ribbon presentation of pore forming S6 helices for the threonine mutant channel. Note that helix IIIS6 is distorted in the middle, while the rest of the helix remains helical throughout the simulation. Snapshot after 300 ps of unconstrained MD. (D) Details of amino acid interactions in the pore forming helices of segment II of the wild-type channel. Hydrophobic interactions between L786 (IIS6) and I1190 (IIIS6) are lost and I1190 is facing towards the opposite side, as a consequence of the I781T mutation.