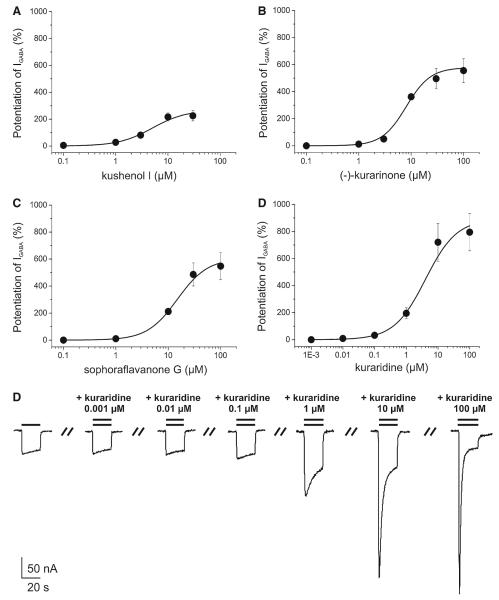
Fig. 7.
Concentration–response curves for IGABA enhancement by a kushenol I (EC50 = 5.0 ± 2.3 μM, nH = 1.3 ± 0.3, n = 5); b (–)-kurarinone (EC50 = 8.1±1.4 μM, nH = 1.9±0.1, n = 4); c sophoraflavanone G (EC50 = 15.0±3.6 μM, nH = 1.5±0.2, n = 3); and d kuraridine (EC50 = 4.0±2.4 μM, nH = 0.9±0.1) in Xenopus oocytes expressing GABAA receptors composed of α1,β2, and γ2S subunits. A maximum potentiation of IGABA by kushenol I (a, 267.6 ± 56.6%, n = 5), (–)-kurarinone (b, 578.5 ± 68.8%, n = 4), sophoraflavanone G (c, 604.9 ± 108.2%, n = 3), kuraridine (d, 891.5 ± 163.0%, n = 3) was observed. e Representative currents through α1β2γ2S GABAA receptors in the presence of GABA (EC3–10, single bar, control) and currents recorded during co-application of GABA (EC3–10) and 0.001, 0.01, 0.1, 1, 10, and 100 μM of kuraridine (double bar)