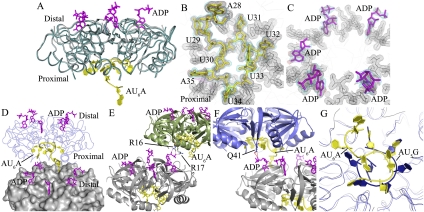
Figure 2.
Global structure of the Hfq–AU6A–ADP complex. (A) Each asymmetric unit contains one Hfq hexamer (dark-cyan ribbon), five ADPs (purple stick), and one AU6A molecule (yellow cartoon). The apo Hfq (gray ribbon) structure was not significantly affected by the binding of AU6A RNA and ADP. The apo Ec Hfq was fitted to bond Hfq in the Hfq65–AU6A–ADP complex using PyMol. (B,C) Electron density maps of AU6A and ADP, respectively. AU6A RNA is in yellow, and ADP is in purple. Hfq is represented as gray lines. Electron density of Hfq residues involved in RNA binding is shown as a gray mesh. Difference maps of Fo–Fc contoured at 2.2σ before inclusion of RNA and ADP are shown in red. Densities (2Fo–Fc) contoured at 1.0σ are shown in cyan. (D) Two closely packed asymmetric units were put together; one Hfq hexamer is presented as molecular surface and colored gray, and the other is presented as a ribbon in blue. ADP and AU6A are represented as sticks. Five out of six R sites on the distal side are occupied by ADPs, while the remaining R site is occupied by the 5′-end adenosine of AU6A (A28 on DsrA), which is bound mostly on the proximal side of another Hfq hexamer. (E) ADPs bind to R sites on the distal side of one Hfq hexamer and positions phosphate groups above the distal side. The α and β phosphates of one ADP on one Hfq hexamer (gray) contact the side chains of R16 and R17 on the proximal side of another Hfq hexamer (green). (F) The α phosphates of one ADP on one Hfq hexamer (gray) contact the side chains of Q41 on the proximal side of another Hfq hexamer (blue). (G) Superposition of the Ec Hfq–AU6A–ADP and Sa Hfq–AU5G complex structures. AU6A (yellow cartoon) recognition by the Ec Hfq (gray ribbon) proximal side differs from AU5G (blue cartoon) recognition in the Sa Hfq (blue ribbon)–AU5G complex.