Figure 3.
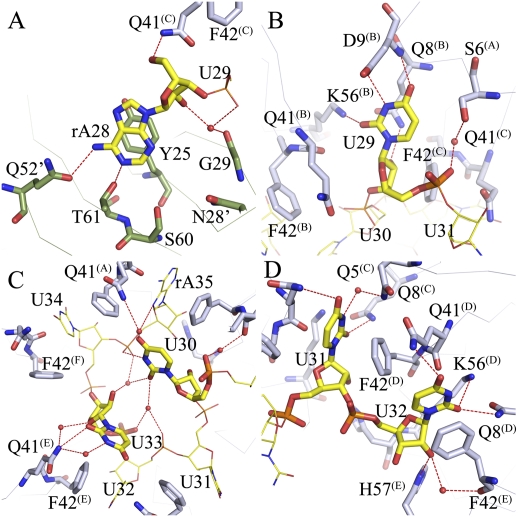
Binding of AU6A to Hfq is unique. (A) The 5′-end adenosine of AU6A, A28, binds to the R site on the distal side of an adjacent Hfq hexamer, stacking against the side chains of Y25 and forming polar contacts with Q52, T61, S60, N28, and G29. Adenines on poly(A) bind to R sites in a similar way. (B) U29 binds to a novel uridine recognition site on the proximal side. Instead of stacking against the side chains of F42 and Q41, U29 uracil O3, O4, and N2 forms polar contacts to the D9, Q8, and K56 side chains. The uracil lies in a pocket in the vicinity of the N termini of the N-terminal α helix. (C) U30 and U33 sit above the proximal side of the central pore, making little contact with Hfq. These two bases are coordinated by a network of water-mediated hydrogen bonds. The interactions between these two nucleic acid residues may be the cause of usual conformation. (D) Canonical binding pattern at the proximal site. U31, U32, U34, and A35 bind in a way similar to that observed in the Sa AU5G Hfq complex.