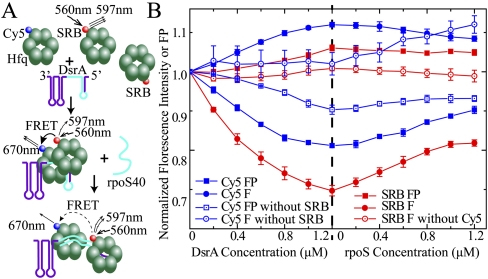
Figure 6.
Different HfqFL hexamers cooperate in binding to DsrA sRNA. (A) Cy5 maleimide was site-specifically labeled to C6 of HfqFL S6C, and SRB was coupled to the lysine side chain. SRB could conjugate to all exposed lysines on Hfq. About 1.2:1 dye:Hfq hexamer labeling efficiency, on average, for both labeled proteins was obtained. The excitation wavelength was 560 nm, and the emission maximum of SRB and Cy5 were 597 nm and 670 nm, respectively. When different Hfq hexamers cooperate to bind one single DsrA, the FRET between SRB and Cy5 was observed due to approximation of different hexamers. rpoS40 constitutes the DsrA pairing region on rpoS mRNA. The rpoS40 and rpoS pairing region on DsrA is colored cyan. Upon annealing of rpoS40 to DsrA, the interaction of Hfq–DsrA is destabilized, resulting in an increase in Hfq hexamer distance. (B) SRB-labeled Hfq (0.6 μM) was mixed with 1.2 μM Cy5-labeled Hfq to give a total Hfq concentration of 1.8 μM. Fluorescence intensity (F, filled circles) and FP (filled rectangles) of both SRB (red) and Cy5 (blue) during titration of DsrA and, subsequently, rpoS40 were recorded. Control was made by mixing 0.6 μM SRB-labeled Hfq (or nonlabeled Hfq) with 1.2 μM nonlabeled HfqFL (or Cy5-labeled HfqFL, excited at 645 nm with the SRB-free sample). (Open circle) Fluorescence intensity; (open rectangles) FP. A decrease of donor fluorescence (SRB) and acceptor fluorescence (Cy5) polarization accompanied by an increase of acceptor fluorescence demonstrates energy transfer between the two fluorophores upon DsrA binding. Annealing of rpoS40 to DsrA partially reversed the FRET effect induced by DsrA.