Abstract
Previous studies have demonstrated that the metabolic syndrome is associated with impaired skeletal muscle arteriolar function, although integrating observations into a conceptual framework for impaired perfusion in peripheral vascular disease (PVD) has been limited. This study builds on previous work to evaluate in situ arteriolar hemodynamics in cremaster muscle of obese Zucker rats (OZR) to integrate existing knowledge into a greater understanding of impaired skeletal muscle perfusion. In OZR cremaster muscle, perfusion distribution at microvascular bifurcations (γ) was consistently more heterogeneous than in controls. However, while consistent, the underlying mechanistic contributors were spatially divergent as altered adrenergic constriction was the major contributor to altered γ at proximal microvascular bifurcations, with a steady decay with distance, while endothelial dysfunction was a stronger contributor in distal bifurcations with no discernible role proximally. Using measured values of γ, we found that simulations predict that successive alterations to γ in OZR caused more heterogeneous perfusion distribution in distal arterioles than in controls, an effect that could only be rectified by combined adrenoreceptor blockade and improvements to endothelial dysfunction. Intravascular 125I-labeled albumin tracer washout from in situ gastrocnemius muscle of OZR provided independent support for these observations, indicating increased perfusion heterogeneity that was corrected only by combined adrenoreceptor blockade and improved endothelial function. These results suggest that a defining element of PVD in the metabolic syndrome may be an altered γ at microvascular bifurcations, that its contributors are heterogeneous and spatially distinct, and that interventions to rectify this negative outcome must take a new conceptual framework into account.
Keywords: rodent models of obesity, microcirculation, skeletal muscle blood flow regulation, models of peripheral vascular disease, blood flow heterogeneity, vascular dysfunction
for myriad social, biological and behavioral reasons (14, 26), Western societies and economies are experiencing a robust growth in the incidence and prevalence of obesity, as well as impaired glycemic control (both from insulin resistance and the more severe type II diabetes mellitus). While there are multiple venues through which these conditions can impact public health, patient quality of life and mortality (13, 26), one of the most common is via an elevated risk for the development of peripheral vascular disease (PVD), which can generally be considered as an evolving condition, wherein the ability of the vasculature to effectively deliver and distribute blood perfusion relative to local metabolic demand becomes increasingly compromised. While the presence of PVD based on structural alterations to larger conduit arteries, including atherosclerotic plaques/lesions and other structural abnormalities that intrude on the vascular lumen represents a major contributor to the overall prevalence of PVD (1, 9), the presence of an array of “microvasculopathies” that are not a function of structural hindrances to perfusion can also represent a powerful contributor to a growing PVD under “nonatherosclerotic” conditions (17, 34).
In the obese Zucker rat (OZR), classically considered as a model of the metabolic syndrome based on chronic hyperphagia subsequent to a disruption of leptin signaling at its receptor, we (16, 19) and others (40, 41) have demonstrated that basal perfusion and/or functional hyperemia can be compromised through adrenergically based elevations in vascular resistance (19, 35) or increased adrenergic signaling (31), alterations to vascular endothelial function that can impact myogenic activation (21) and multiple parameters of dilator responses (7, 8, 16, 23, 25, 37). However, an understanding of how this array of contributors to an integrated vascular/perfusion-based outcome relevant for skeletal muscle performance or fatigue resistance has proven to be an exceptionally difficult process, owing to difficulties in translating results in isolated tissues/ex vivo preparations to the functioning intact system, the inherent complexity of studying multiple processes simultaneously and the challenges of accurately integrating the concepts of space (location) and time into system behaviors and outcomes.
In our recent studies, we have provided evidence that the ability of in situ skeletal muscle of OZR to resist fatigue under conditions of elevated metabolic demand is partially a function of the inability to reduce the aggregate vascular resistance through the microvascular network (which was largely dependent on reducing adrenergic constrictor tone) and elevate oxygen extraction and conductance within the distal microcirculation (which was predominantly related to elevated vascular oxidant stress and the effects of the increased vascular production of thromboxane A2, TxA2) (15, 16). While alterations to skeletal muscle performance with either adrenoreceptor blockade or antioxidant treatment/PGH2/TxA2 receptor antagonism were trivial, the combination of these interventions effectively resulted in a maximal improvement to perfusion, O2 extraction, O2 conductance (a lumped parameter describing skeletal muscle oxygen flux that reflects all of the resistances for O2 in moving from red blood cell to muscle fiber mitochondria; Ref. 38), resulting in significant improvements to muscle oxygen uptake (V̇o2) and contractile performance. These observations, which help to identify the specific role of microvascular dysfunction in limiting V̇o2 and muscle performance at low, moderate, and near-maximal metabolic demand, are especially intriguing, given the results from previous studies, which suggested that one of the defining characteristics of PVD and a poor perfusion outcome in OZR is an increasingly heterogeneous blood flow distribution at microvascular bifurcations within skeletal muscle (16). While that study provided an initial insight revealing the presence of increased perfusion heterogeneity at bifurcations, those results focused on only the distal microcirculation and provided no insight into either the presence of perfusion heterogeneity at different levels of the microcirculation or how contributing elements to this effect might vary with longitudinal position within the networks (16). In addition, those previous data provided no information regarding the functional outcomes or implications that result from the increased perfusion heterogeneity.
The primary purpose of the present study was to interrogate the presence of the increased perfusion heterogeneity at four distinct levels of the skeletal muscle microcirculation of OZR, to determine the physiological mechanistic contributors to this process (and if they vary with position within the microcirculation), and to predict the functional implications of this process for perfusion patterns within the distal levels of the microcirculation. A secondary purpose of the present study was to use indicator dilution analyses in intact skeletal muscle to determine the presence and impact of increased microvascular perfusion heterogeneity in skeletal muscle of OZR and the role of the physiological mechanisms determined at the higher level of resolution (individual bifurcations) in contributing to these outcomes. Specifically, these studies tested the hypothesis that the presence of increased heterogeneity of perfusion distribution at successive bifurcations within the skeletal muscle microcirculation results in severe impairments to distal arteriolar blood flow distribution and is a major contributor to the impaired muscle performance outcomes that have been previously demonstrated in OZR (15, 20).
MATERIALS AND METHODS
Animals.
Male lean Zucker rats (LZR; total n = 26) and obese Zucker rats (total n = 102), purchased from Harlan (Madison, WI), were fed standard chow and drinking water ad libitum and were housed in the animal care facility at the West Virginia University Health Sciences Center or the Medical College of Wisconsin. All protocols received prior Institutional Animal Care and Use Committee approval. At ∼17 wk of age, rats were anesthetized with injections of pentobarbital sodium (50 mg/kg ip) and received tracheal intubation to facilitate maintenance of a patent airway. In all rats, a carotid artery and an external jugular vein were cannulated for determination of arterial pressure and for infusion of supplemental anesthetic and pharmacological agents, as necessary. Any animal in which mean arterial pressure (MAP) was found to be below 85 mmHg, or where MAP had decreased by more than 15% from that following equilibration (without any pharmacological intervention) was not used in the present study. Blood samples were drawn from the venous cannula for determination of glucose and insulin concentrations (Millipore, Billerica, MA), as well as cholesterol/triglyceride levels (Wako Diagnostics, Richmond, VA), and nitrotyrosine (Oxis International, Foster City, CA). Unless otherwise noted, all drugs and chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of in situ cremaster muscle.
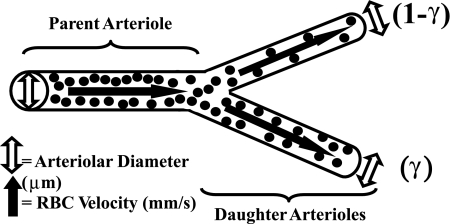
In each rat (LZR n = 16; OZR n = 74), an in situ cremaster muscle was prepared for study using intravital microscopy, as described previously (16). After completion of the in situ cremaster muscle preparation, the tissue was continuously superfused with physiological salt solution (PSS) equilibrated with a 5% CO2-95% N2 gas mixture, and maintained at 35°C, as it flowed over the muscle. Volume flow rate was ∼3.0 ml/min. The ionic composition of the PSS was as follows (in mM): 119.0 NaCl, 47 KCl, 1.6 CaCl2, 1.18 NaH2PO4, 1.17 MgSO4, 24.0 NaHCO3, and 0.03 disodium EDTA. Arteriolar diameters were measured with a video micrometer. Following a 30-min period of equilibration following the surgical preparation, arterioles and bifurcations within five distinct diameter categories were selected for investigation; ∼100 μm (1A), ∼80 μm (2A), ∼60 μm (3A), ∼40 μm (4A), and ∼20 μm (5A) in diameter. This resulted in four “bifurcation categories”: 1) ∼100-μm “parent” to ∼80-μm “daughters” (1A-2A), 2) ∼80-μm parent to ∼60-μm daughters (2A-3A), 3) ∼60-μm parent to ∼40-μm daughters (3A-4A), and 4) ∼40-μm parent to ∼20-μm daughters (4A–5A). Arterioles/bifurcations were selected based on the following criteria: 1) distance from any site of incision, 2) presence of significant vascular tone (assessed by brisk dilator response to challenge with 10−3 M adenosine), 3) clearly discernible walls, 4) a rapid and stable level of erythrocyte perfusion, and 5) the presence of two clearly defined daughter branches that also met criteria 1–4. Please see Fig. 1 for a schematic representation of these concepts.
Fig. 1.
Schematic representation of the in situ cremasteric arteriolar bifurcation used for assessing “parent” and “daughter” arteriolar mechanical and hemodynamic/perfusion responses to pharmacological challenge. Open arrows represent parent or daughter arteriolar diameter in response to a specific condition; solid arrows represent parent or daughter arteriolar erythrocyte velocity in response to a specific challenge. These data are utilized to determine both arteriolar flow volume and perfusion heterogeneity at bifurcations (γ). Please see text for additional detail.
The mechanical (using on-screen video microscopy) and perfusion (using optical Doppler velocimetry) responses of both the parent and daughter arterioles within a bifurcation class were assessed under resting conditions within the cremaster muscle of each rat. All procedures were performed under control conditions in LZR and OZR, and following treatment of the in situ cremaster muscle with the antioxidant TEMPOL (10−3 M), the TxA2 receptor antagonist SQ-29548 (10−4 M), the nitric oxide synthase inhibitor l-NAME (10−4M), and/or the α1/α2 adrenergic receptor antagonist phentolamine (10−5 M); within the superfusate solution. All treatments to the cremaster were of a minimum of 40 min duration prior to any subsequent experimental manipulation.
Interrogation of arteriolar bifurcations.
Within each animal, the numbers of bifurcations of each order that were studied differed depending on longitudinal position in the arteriolar network. Bifurcations spanning 1A–2A arterioles averaged 1 or 2 per animal, while bifurcations spanning 3A–4A and 4A–5A arterioles averaged between 3 and 4 per animal. For an individual measurement, arteriolar diameter and erythrocyte velocity were sampled for 10 s. All arterioles and bifurcations were selected on the basis of the criteria outlined above and were placed into their categories by size rather than by strict branch number. This was done as our experience demonstrated that branch order classification limited data collection, as frequently not all inclusion criteria could be satisfied.
No cremaster muscle was exposed to all interventions, as this would have compromised data quality owing to experiments of excessive duration. In addition to the collection of responses under control conditions, individual cremaster preparations were exposed to a maximum of three interventions, each separated by ∼30 min of washout. Treatment or washout effectiveness was verified by determining abolition or recovery of mechanical responses following challenge with appropriate agonists (e.g., the α1 adrenergic antagonist phenylephrine, the stable TxA2 mimetic, U-46619, and the endothelium-dependent dilator agonist, ACh). Maximum experimental duration from preparation to termination was approximately 5 h, after which time, all animals were humanely euthanized by an intravenous overdose of anesthetic followed by a bilateral pneumothoracotomy.
In the present study, blood flow within a parent arteriole was measured directly, with γ reflecting the proportion in the daughter arteriole with the higher perfusion. Within an individual arteriolar bifurcation, the maximum disparity between parent arteriole blood flow and the summated daughter arteriolar perfusion did not exceed 15%. If this error were consistent in terms of magnitude and direction, this could lead to a biased outcome on the distal arteriolar perfusion distributions. However, there is no a priori reason to believe that this would be a consistent error in terms of artificially increasing or decreasing Q determination, and it is more likely that measurement errors would be randomly distributed. To the extent possible, all measurements within an experiment were taken at the same arteriolar bifurcation sites within a specific animal. However, this was not possible in every animal, owing to alterations to visibility that can develop with time and interventions in this experimental preparation.
Preparation of in situ blood perfused hindlimb.
In a separate set of age-matched LZR (n = 10) and OZR (n = 28), the left hindlimb of each animal was isolated in situ (20) with minor modifications. Heparin (500 IU/kg) was infused via the jugular vein to prevent blood coagulation. Subsequently, an angiocatheter was inserted into the femoral artery, proximal to the origin of the gastrocnemius muscle to allow for bolus tracer injection. Additionally, a small shunt was placed in the femoral vein draining the gastrocnemius muscle that allowed for diversion of flow into a port that facilitated sampling of the venous effluent. Finally, a microcirculation flow probe (Transonic, Ithaca, NY; 0.5/0.7 PS) was placed on the femoral artery to monitor muscle perfusion. In individual experiments, rats received intravenous infusion of the α1/α2 adrenoreceptor antagonist phentolamine (10 mg/kg), TEMPOL (50 mg/kg), and/or SQ-29548 (10 mg/kg). Effectiveness of these interventions was assessed by monitoring the changes in arterial pressure in response to intravenous infusion of phenylephrine (10 μg/kg), methacholine (10 μg/kg) or U-46619 (10 μg/kg), respectively (15, 16).
Upon completion of the surgical preparation, the gastrocnemius muscle was allowed 30 min of self-perfused rest. At this point, 20 μl of 125I-labeled albumin (10 μCi; Perkin-Elmer, Shelton, CT) was injected as a spike bolus (injection time <0.5 s) into the arterial angiocatheter, and venous effluent samples were collected at a rate of 1/s for the subsequent 35 s. Venous effluent samples were then immediately transferred into silicate tubes and placed into a gamma counter for activity determination. Each rat received an intravenous infusion of homologous donor erythrocytes suspended in PSS at the individual animal's hematocrit (∼45%) to replace the lost volume, and this was allowed a minimum of 20 min for circulation prior to subsequent intervention. To assess the potential for leakage of the labeled albumin from the intravascular space as a source for error, the gastrocnemius muscle was cleared by perfusion with physiological salt solution following euthanasia. Subsequent to a determination of mass, the muscle was placed in the counter for determination of residual activity. Residual activity within the gastrocnemius muscle did not exceed 200 cpm/animal, a level that was far lower than those determined in the venous blood aliquots. All protocols had received prior approval from the Radiation Safety Office at the West Virginia University Health Science Center.
Data and statistical analyses.
Arteriolar perfusion in both parent and daughter vessels within in situ cremaster muscle of LZR and OZR was calculated as
| (1) |
where Q represents arteriolar perfusion (nl/s), V represents the measured red blood cell velocity from the optical Doppler velocimeter (mm/s; with V/1.6 representing an estimated average velocity; Ref. 11), and r represents arteriolar radius (μm; Ref. 3). The total volume perfusion in the daughters was determined as the sum of the individual perfusion rates, and the proportion of flow within each was determined as the quotient of the individual branch divided by the total. γ is defined as the ratio of the greater of the two flows in the daughter vessel to the total flow in the parent vessel. As an example, if flow distribution were homogeneous between daughters, γ for that bifurcation would be 0.5 in both daughter arterioles, while if the proportion of flow in one daughter arteriole were 60%, γ for that bifurcation would be 0.6, with flow distribution being 0.6 in the high-perfusion arteriole and 0.4 in the low-perfusion arteriole (5). Please see Fig. 1 for additional clarification.
To predict the outcomes of the successive levels of γ for distal microvascular perfusion distribution in the distal microcirculation, the individual measured levels of γ from the experiments using the cremaster muscle were inserted into a simulation of a dichotomous branching network with each outflow (daughter) arteriole arising from an inflow (parent) arteriole receiving either γ or (1-γ) as the distribution of perfusion. These respective values of daughter arteriolar perfusion then become in the inflow perfusion (parent) values for the next-generation bifurcation. The process was repeated over a total of eight bifurcations, resulting in 28 or 256 parallel arterioles. This simulation used the measured value of γ for the 1A–2A, the 2A–3A values of γ for the next two generations, the 3A–4A values of γ for the next two generations, and three 4A–5A values of γ to generate flows in the final three generations.
For the 125I-labeled albumin washout, four standard parameters describing characteristics of tracer washout curves, including mean transit time (t̄), relative dispersion (RD), skewness (β1), and kurtosis (β2), were computed as functions of the transport function h(t) (6). The tail of tracer washout curves is extrapolated in the form of single exponential time course to allow for computing the four parameters by the integration for a sufficiently long time for Eq. 3 to converge (24). In this study, experimentally measured time courses are extrapolated to 100 s, at which C(t) is estimated to be less than 10−9 of the maximum washout tracer activity. The transport function h(t) is estimated from
| (2) |
where C(t) is the time course of activity of intravascular tracer in outlet flow exiting the collecting tube. The mean transit time, t̄, is calculated from experimentally measured washout curves according to
| (3) |
The RD of h(t) is a measure of the relative temporal spread of h(t) and computed as the ratio of standard deviation of h(t) to the mean transit time from
| (4) |
The skewness (β1) is a measure of asymmetry of h(t) and computed from
| (5) |
Skewness is a measure of the asymmetry of the perfusion distribution. In other words, it is a measure of the extent to which the perfusion distribution is skewed (as opposed to simply being shifted) to higher or lower values of perfusion. The kurtosis (β2) is a measure of deviation of h(t) from a normal distribution and computed from
| (6) |
Kurtosis is a measure of the “sharpness of the peak” of the perfusion distribution. The familiar Gaussian bell-shaped curve has β2 = 0; positive values of β2 indicate a sharper peak than a Gaussian curve. The four parameters are estimated on the basis of the above equations for each animal in each experimental group.
All data throughout the manuscript are presented as means ± SE. Statistically significant differences in measured and calculated parameters were determined using a one-sample t-test (differences from zero), Student's t-test or ANOVA with Student-Newman-Keuls post hoc test used as needed. Analyses of the simulation results for distal arteriolar perfusion distribution (Fig. 6) used descriptive statistics [variance, skewness, kurtosis, range, maximum, minimum, quartiles (25%, 50%, and 75%) and the 75–25% interquartile difference]. Statistically significant differences between the frequency distributions for the simulations were evaluated using the Kruskal-Wallis test, although because indices of central tendency were not different between groups (due to the normalization procedures), multiple post hoc comparisons were not possible. In all cases, P < 0.05 was taken to reflect statistical significance.
Fig. 6.
Predicted perfusion distributions in the distal microcirculation of skeletal muscle of LZR and OZR under the conditions of the present study. Frequency distributions are calculated on the basis of an eight bifurcation network using the microvascular perfusion distribution coefficients (γ) determined in the in situ cremaster muscle presented in Figs. 2–5. Individual panels present the distribution of perfusion across 256 (28) parallel arterioles under each experimental condition as a result of the simulation of a dichotomous branching network. Please see text for additional detail.
RESULTS
Baseline characteristics describing the animals used in the present study are summarized in Table 1. At ∼17 wk of age, male OZR were significantly heavier than LZR and, in addition to a moderate hypertension, hypercholesterolemia, and hyperglycemia, also exhibited a severe elevation in plasma levels of insulin, triglycerides, and nitrotyrosine. Data describing the perfusion characteristics of the individual cremasteric arteriolar segments (described above) under the experimental conditions of the present study are summarized in Table 2. These data clearly demonstrate general differences between arteriolar diameter and perfusion characteristics that are distributed longitudinally throughout the cremasteric microcirculation under the conditions of the present study.
Table 1.
Baseline characteristics of approximately 17-wk-old LZR and OZR used in the present study
| LZR (n = 26) | OZR (n = 102) | |
|---|---|---|
| Mass, g | 366 ± 8 | 684 ± 11* |
| MAP, mmHg | 101 ± 5 | 124 ± 6* |
| Glucoseplasma, mg/dl | 111 ± 10 | 184 ± 14* |
| Insulinplasma, ng/ml | 2.0 ± 0.4 | 9.4 ± 1.1* |
| Cholesterolplasma, mg/dl | 78 ± 9 | 138 ± 12* |
| Triglyceridesplasma, mg/dl | 84 ± 11 | 348 ± 19* |
| Nitrotyrosineplasma, ng/ml | 14 ± 4 | 48 ± 8* |
Data are presented as means ± SE. LZR, lean Zucker rats; OZR, obese Zucker rats; MAP, mean arterial pressure.
P < 0.05 vs. LZR.
Table 2.
Arteriolar perfusion in LZR and OZR
| LZR | OZR | O-P | O-T | O-SQ | O-LN | O-PT | O-PSQ | O-PLN | O-TLN | O-TSQ | O-PTSQ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | ||||||||||||
| ID | 94 ± 4 | 85 ± 6 | 92 ± 3 | 87 ± 6 | 88 ± 5 | 84 ± 6 | 93 ± 3 | 93 ± 4 | 90 ± 4 | 86 ± 3 | 86 ± 4 | 93 ± 3 |
| VRBC | 56 ± 5 | 46 ± 6 | 48 ± 5 | 45 ± 6 | 49 ± 7 | 38 ± 6* | 52 ± 6 | 53 ± 5 | 46 ± 5 | 48 ± 5 | 48 ± 4 | 53 ± 5 |
| Q | 245 ± 14 | 165 ± 20* | 200 ± 15 | 169 ± 14* | 186 ± 9* | 134 ± 15* | 219 ± 6† | 224 ± 14† | 183 ± 13* | 175 ± 13* | 176 ± 14* | 227 ± 17† |
| 2A | ||||||||||||
| ID | 75 ± 4 | 66 ± 5 | 72 ± 3 | 69 ± 6 | 68 ± 6 | 64 ± 5 | 73 ± 4 | 74 ± 4 | 71 ± 5 | 67 ± 6 | 69 ± 5 | 74 ± 4 |
| VRBC | 40 ± 6 | 35 ± 5 | 40 ± 5 | 36 ± 6 | 36 ± 5 | 32 ± 4 | 39 ± 5 | 39 ± 4 | 36 ± 6 | 37 ± 5 | 38 ± 6 | 40 ± 4 |
| Q | 112 ± 10 | 74 ± 12* | 100 ± 9 | 84 ± 9* | 82 ± 5* | 65 ± 6* | 102 ± 5 | 105 ± 8 | 89 ± 9 | 82 ± 11* | 89 ± 12 | 108 ± 6† |
| 3A | ||||||||||||
| ID | 53 ± 4 | 48 ± 7 | 50 ± 5 | 53 ± 6 | 53 ± 5 | 47 ± 6 | 52 ± 5 | 53 ± 5 | 48 ± 5 | 52 ± 5 | 53 ± 6 | 53 ± 5 |
| VRBC | 20 ± 4 | 14 ± 3 | 17 ± 4 | 14 ± 3 | 14 ± 3 | 12 ± 3 | 18 ± 3 | 18 ± 4 | 15 ± 4 | 12 ± 3 | 14 ± 4 | 20 ± 2 |
| Q | 28 ± 4 | 16 ± 4* | 21 ± 3 | 19 ± 4 | 19 ± 3 | 13 ± 3* | 24 ± 5 | 25 ± 5 | 17 ± 3 | 16 ± 3 | 19 ± 4 | 28 ± 4* |
| 4A | ||||||||||||
| ID | 36 ± 4 | 30 ± 3 | 31 ± 3 | 33 ± 5 | 32 ± 4 | 28 ± 3 | 34 ± 5 | 34 ± 4 | 29 ± 4 | 32 ± 5 | 35 ± 4 | 35 ± 3 |
| VRBC | 12 ± 3 | 8 ± 2 | 12 ± 3 | 9 ± 2 | 10 ± 3 | 8 ± 2 | 11 ± 2 | 12 ± 2 | 8 ± 2 | 9 ± 2 | 9 ± 2 | 12 ± 2 |
| Q | 7.8 ± 1.9 | 3.7 ± 1.5 | 5.5 ± 1.7 | 4.7 ± 1.1 | 4.8 ± 1.3 | 3.1 ± 0.8* | 6.1 ± 1.6 | 6.6 ± 1.3 | 3.5 ± 0.9 | 4.3 ± 1.2 | 5.5 ± 1.4 | 7.2 ± 1.6 |
| 5A | ||||||||||||
| ID | 21 ± 3 | 18 ± 3 | 18 ± 2 | 20 ± 3 | 19 ± 3 | 18 ± 3 | 19 ± 2 | 20 ± 3 | 18 ± 2 | 19 ± 3 | 19 ± 2 | 20 ± 2 |
| VRBC | 7 ± 1 | 4 ± 1 | 5 ± 2 | 4 ± 1 | 4 ± 1 | 3 ± 1 | 6 ± 2 | 7 ± 2 | 5 ± 2 | 5 ± 2 | 5 ± 1 | 7 ± 1 |
| Q | 1.5 ± 0.3 | 0.7 ± 0.2* | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.2* | 0.5 ± 0.2* | 1.1 ± 0.3 | 1.3 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.3 | 1.4 ± 0.3 |
Data are presented as means ± SE. Data are presented for arteriolar inner diameter (ID; μm), centerline erythrocyte velocity (VRBC; mm/s), and volume perfusion within the arteriole (Q; nl/s).
P < 0.05 vs. LZR;
P < 0.05 vs. OZR. P denotes treatment with phentolamine; T denotes treatment with TEMPOL; SQ denotes treatment with SQ-29548; LN denotes treatment with l-NAME; with combinations representing treatment with multiple agents.
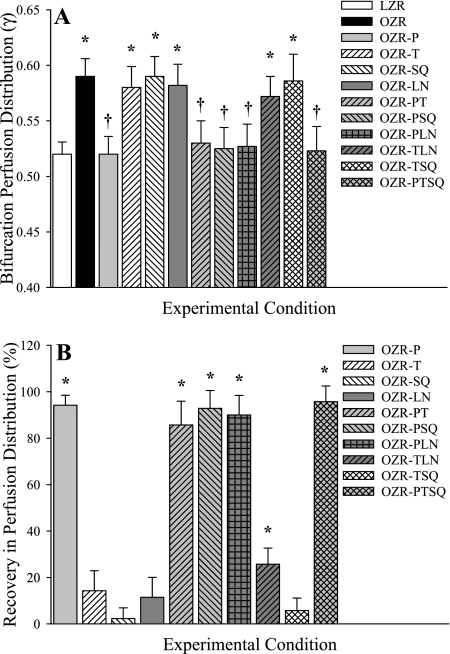
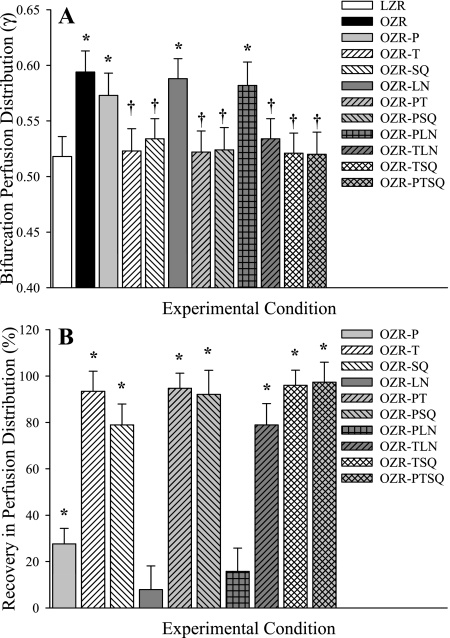
Figure 2 presents the magnitude of the perfusion distribution coefficient (γ) at bifurcations spanning 1A (100 μm) parent arterioles to 2A (80 μm) daughter arterioles in LZR and OZR under control conditions and in response to the interventions employed in the present study (Fig. 2A) and the extent to which specific interventions restore γ in OZR to levels determined in LZR (Fig. 2B). Specifically, the ordinate in Fig. 2B is defined as:
| (7) |
where γOZR−* represents one of the treatments indicated. Full (100%) recovery indicates that the indicated treatment restores γ to the LZR value. Zero (0%) recovery indicates that the treatment has no effect on γ.
Fig. 2.
Microvascular perfusion distribution (γ) at bifurcations spanning 1A (parent) and 2A (daughter) arterioles within in situ cremaster muscle. Data are presented as means ± SE for lean Zucker rats (LZR) under control conditions and in obese Zucker rats (OZR) under control conditions and following treatment with the adrenoreceptor antagonist phentolamine (P), the antioxidant TEMPOL (T), the PGH2/TxA2 receptor blocker SQ-29548 (SQ), the nitric oxide synthase inhibitor l-NAME (LN), or combinations of these agents. A: data describing the magnitude of γ at the 1A-2A bifurcation under the specific experimental conditions. B: % recovery (to the level determined in LZR) in γ at that bifurcation as a result of the imposed pharmacological challenge. Please see text for additional detail. A, *P < 0.05 vs. LZR; †P < 0.05 vs. OZR. B, *P < 0.05 vs. no change.
In these proximal resistance arterioles, γ was significantly elevated in OZR compared with LZR, although this was restored by treatment of the cremaster muscle with the adrenoreceptor antagonist phentolamine (either alone or in combination with any other agent). In contrast, treatment with TEMPOL, SQ-29548, or l-NAME, either alone or in any combination, was without effect in terms of restoring the normal magnitude of γ unless phentolamine was also present.
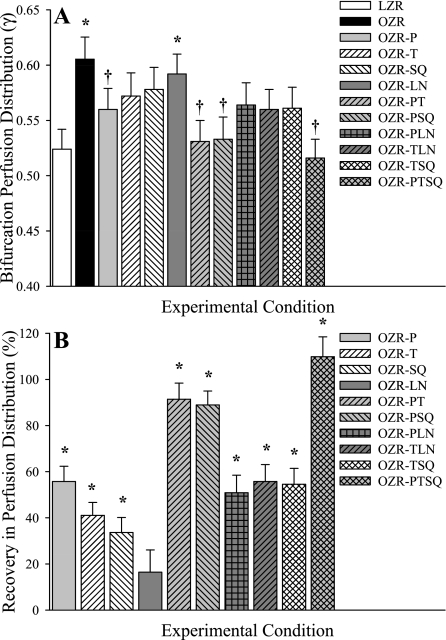
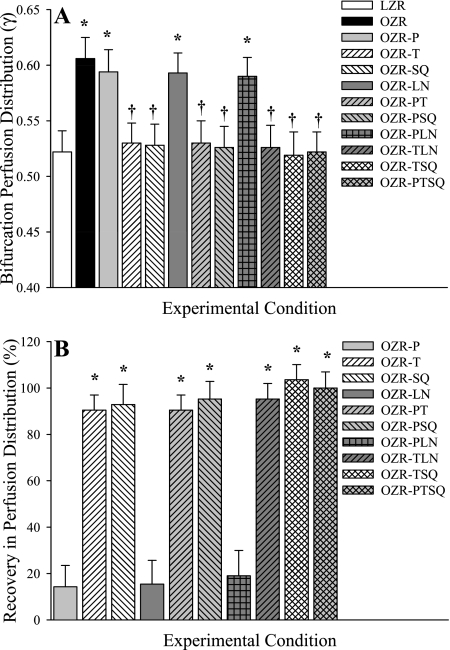
The magnitude of γ (Fig. 3A) and its recovery following intervention (Fig. 3B), in arteriolar bifurcations spanning 2A (80 μm) parent to 3A (60 μm) daughter arterioles in cremaster muscle of LZR and OZR are summarized. In contrast to the data presented in Fig. 2, the ability of phentolamine to restore normal levels of γ in OZR at this level of the cremasteric microcirculation, while still statistically significant, was blunted compared with responses at the more proximal bifurcation. Neither TEMPOL nor SQ-29548 elicited a significant improvement in γ at this level of the microcirculation when applied individually. Further, when either TEMPOL or SQ-29548 was coadministered with phentolamine, these restorative effects appeared to be partially additive, as the improvement to γ was substantially elevated compared with any single intervention. Interestingly, l-NAME treatment alone did not result in a significant change to γ in OZR, and this was not impacted by amelioration of vascular oxidant stress by coadministration with TEMPOL.
Fig. 3.
Microvascular perfusion distribution (γ) at bifurcations spanning 2A (parent) and 3A (daughter) arterioles within in situ cremaster muscle. Data are presented as means ± SE for LZR under control conditions and in OZR under control conditions and following treatment with the adrenoreceptor antagonist phentolamine (P), the antioxidant TEMPOL (T), the PGH2/TxA2 receptor blocker SQ-29548 (SQ), the nitric oxide synthase inhibitor l-NAME (LN), or combinations of these agents. A: data describing the magnitude of γ at the 2A-3A bifurcation under the specific experimental conditions. B: % recovery (to the level determined in LZR) in γ at that bifurcation as a result of the imposed pharmacological challenge. Please see text for additional detail. A, *P < 0.05 vs. LZR; †P < 0.05 vs. OZR. B, *P < 0.05 vs. no change.
The magnitude of γ (A), and its recovery following intervention (B), in 3A (60 μm) parent to 4A (40 μm) and 4A to 5A (20 μm) daughter arteriolar bifurcations in cremaster muscle of LZR and OZR are presented in Figs. 4 and 5, respectively. At these levels of the cremasteric microcirculation, adrenoreceptor blockade was without significant effect in terms of restoration of γ in OZR to the levels determined in LZR. However, improvements to endothelial function from TEMPOL and SQ-29548, alone or in combination nearly restored the normal levels of γ in the OZR microcirculation at these positions within the networks. As with the data presented above, we were unable to determine an apparent role for nitric oxide bioavailability in terms of regulating γ in the cremasteric microcirculation of OZR.
Fig. 4.
Microvascular perfusion distribution (γ) at bifurcations spanning 3A (parent) and 4A (daughter) arterioles within in situ cremaster muscle. Data are presented as means ± SE for LZR under control conditions and in OZR under control conditions and following treatment with the adrenoreceptor antagonist phentolamine (P), the antioxidant TEMPOL (T), the PGH2/TxA2 receptor blocker SQ-29548 (SQ), the nitric oxide synthase inhibitor l-NAME (LN), or combinations of these agents. A: data describing the magnitude of γ at the 3A-4A bifurcation under the specific experimental conditions. B: % recovery (to the level determined in LZR) in γ at that bifurcation as a result of the imposed pharmacological challenge. Please see text for additional detail. A, *P < 0.05 vs. LZR; †P < 0.05 vs. OZR. B, *P < 0.05 vs. no change.
Fig. 5.
Microvascular perfusion distribution (γ) at bifurcations spanning 4A (parent) and 5A (daughter) arterioles within in situ cremaster muscle. Data are presented as means ± SE for LZR under control conditions and in OZR under control conditions and following treatment with the adrenoreceptor antagonist phentolamine (P), the antioxidant TEMPOL (T), the PGH2/TxA2 receptor blocker SQ-29548 (SQ), the nitric oxide synthase inhibitor l-NAME (LN), or combinations of these agents. A: data describing the magnitude of γ at the 4A-5A bifurcation under the specific experimental conditions. B: % recovery (to the level determined in LZR) in γ at that bifurcation as a result of the imposed pharmacological challenge. Please see text for additional detail. A, *P < 0.05 vs. LZR; †P < 0.05 vs. OZR. B, *P < 0.05 vs. no change.
Using the data presented above for γ distribution at the different levels of the cremasteric microcirculation of LZR and OZR under the employed experimental interventions, Fig. 6 presents the predicted outcomes on perfusion distribution within the terminal arterioles, and Table 3 summarizes the results of the statistical analyses of the distribution outcomes presented in Fig. 6. These predictions are based on a dichotomous branching network of eight bifurcations between the major perfusing arteriole and the terminal arterioles, resulting in a total of 256 terminal arterioles. These data clearly predict that the shifts in γ identified through direct examination of the cremaster muscle result in profound alterations in the outcomes for distal arteriolar perfusion distribution. While the distribution for volume flow in any single arteriole, relative to the mean flow across all 256 arterioles, was relatively tight in LZR compared with OZR (Fig. 6, A vs. B), demonstrating much lower degrees of variability, skewness, and kurtosis, with a much lower interquartile difference (Table 3), treatment with the individual interventions of phentolamine, TEMPOL and SQ-29548 (Fig. 6, C–E, respectively) all results in moderate improvements to the variability, skewness, kurtosis, and interquartile difference of the distributions (Table 3). However, the greatest magnitude of improvement was identified under conditions of adrenoreceptor blockade combined with either TEMPOL or SQ-29548 (or both). These perfusion outcome predictions and statistical analyses also suggest that vascular nitric oxide bioavailability, either under control conditions in OZR skeletal muscle at rest or following reductions to vascular oxidant stress that have been repeatedly demonstrated to increase nitric oxide (NO) bioavailability, may not play a significant role in terms of regulating γ or the distal arteriolar perfusion outcomes.
Table 3.
Statistical analysis of the individual frequency distributions of predicted distal arteriolar perfusion heterogeneity presented in Figure 6
| LZR | OZR | O-P | O-T | O-SQ | O-LN | O-PT | O-PSQ | O-PLN | O-TLN | O-TSQ | O-PTSQ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variance | 0.015 | 0.384 | 0.195 | 0.086 | 0.106 | 0.298 | 0.026 | 0.024 | 0.204 | 0.070 | 0.070 | 0.013 | |
| Skewness | 0.319 | 1.678 | 1.131 | 0.673 | 0.744 | 1.470 | 0.422 | 0.407 | 1.175 | 0.631 | 0.555 | 0.302 | |
| Kurtosis | −0.083 | 4.193 | 1.625 | 0.171 | 0.311 | 3.168 | 0.033 | 0.009 | 1.829 | 0.184 | −0.183 | −0.104 | |
| Range | 0.690 | 4.210 | 2.590 | 1.525 | 1.705 | 3.590 | 0.918 | 0.888 | 2.716 | 1.427 | 1.280 | 0.653 | |
| Minimum | 0.706 | 0.166 | 0.291 | 0.469 | 0.430 | 0.206 | 0.632 | 0.6418 | 0.278 | 0.495 | 0.526 | 0.722 | |
| Maximum | 1.397 | 4.376 | 2.881 | 1.994 | 2.135 | 3.795 | 1.551 | 1.530 | 2.994 | 1.921 | 1.806 | 1.375 | |
| Percentiles | 25 | 0.914 | 0.571 | 0.687 | 0.783 | 0.755 | 0.617 | 0.878 | 0.895 | 0.697 | 0.800 | 0.795 | 0.918 |
| 50 | 0.994 | 0.853 | 0.916 | 0.967 | 0.959 | 0.883 | 0.990 | 0.991 | 0.912 | 0.975 | 0.975 | 0.996 | |
| 75 | 1.081 | 1.275 | 1.223 | 1.194 | 1.216 | 1.263 | 1.117 | 1.098 | 1.194 | 1.187 | 1.195 | 1.082 | |
| IQ Diff. (75–25) | 0.167 | 0.704 | 0.536 | 0.411 | 0.461 | 0.646 | 0.239 | 0.203 | 0.497 | 0.387 | 0.400 | 0.164 |
Data are presented for variance, skewness, kurtosis, and ranges of the individual distributions, as well as the quartiles and the interquartile difference (IQ Diff.). Results from the Kruskal-Wallis test indicate that there are significant differences across the different distributions (P < 0.001). P denotes treatment with phentolamine; T denotes treatment with TEMPOL, SQ denotes treatment with SQ-29548; LN denotes treatment with l-NAME, with combinations representing treatment with multiple agents.
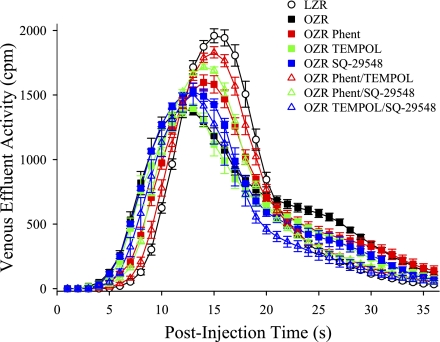
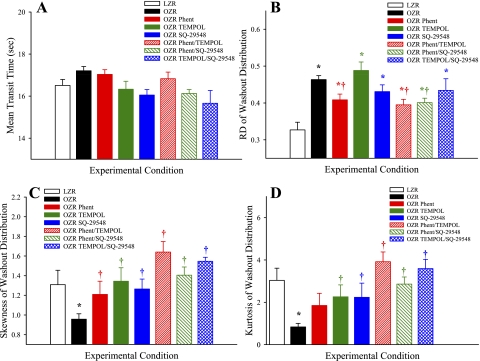
Figure 7 presents the mean tracer washout curves for 125I-labeled albumin from the isolated gastrocnemius muscle of LZR and OZR following the pharmacological treatments described above. Figure 8 summarizes data describing the aggregate washout curves. Mean transit time of the tracer across the gastrocnemius was very similar across all groups, suggesting that the relationship between bulk blood flow to the gastrocnemius muscle and vascular volume under the experimental conditions of the present study were very similar across groups (Fig. 8A). The relative dispersion of tracer across the muscle (RD; Fig. 8B) was significantly elevated in OZR compared with LZR under control conditions, indicative of an increased perfusion heterogeneity throughout the microcirculation of the gastrocnemius muscle. Treatment of OZR with phentolamine (with or without TEMPOL or SQ-29548) significantly improved RD, although treatment with TEMPOL and/or SQ-29548 in the absence of the adrenoreceptor blockade was generally less effective. Both the skewness (Fig. 8C) and the kurtosis (Fig. 8D) of the tracer washout curves were reduced in OZR compared with LZR, and these shifts in the washout patterns were improved toward the level in LZR as a result of the experimental interventions, with the greatest impact being determined in response to combined treatment with phentolamine and TEMPOL/SQ-29548. On the basis of the data presented in Figs. 2–6, this final group of experiments and analyses did not involve direct manipulations to vascular nitric oxide bioavailability.
Fig. 7.
Data describing the washout of 125I-labeled albumin from the in situ gastrocnemius muscle of LZR and OZR under the conditions of the present study. Data are presented as means ± SE for LZR (n = 10) under control conditions and OZR under control conditions (total n = 28) and following intravenous treatment with the adrenoreceptor antagonist phentolamine (P; n = 10), the antioxidant TEMPOL (T; n = 9), the PGH2/TxA2 receptor blocker SQ-29548 (SQ; n = 9), or for combinations of these agents (n = 8–10 for each).
Fig. 8.
Data (presented as means ± SE) describing the four moments of the washout of 125I-labeled albumin from the in situ gastrocnemius muscle of LZR and OZR under the conditions of the present study. Data are shown for the mean transit time of the washout (A), the relative dispersion (RD) of the washout (B), the distribution skewness (C), and kurtosis (D). Please see text for details. *P < 0.05 vs. LZR. †P < 0.05 vs. OZR.
DISCUSSION
A recent study has demonstrated that the increased rate of skeletal muscle fatigue in OZR, defined as the percent decline in developed tension over the duration of the imposed bout of contraction, is not necessarily improved subsequent to an improved functional hyperemic response (15). However, one of the central observations of that study was that the vascular dysfunction in the skeletal muscle microcirculation of OZR impacted muscle performance outcomes through two distinct patterns, such that impairments within the proximal microcirculation restricted volume perfusion, and responses in the distal microcirculation impaired normal patterns of perfusion-demand matching. Further, that study also implicated the possibility that the physiological and cellular mechanisms underlying the vascular dysfunction within the skeletal muscle microcirculation may be spatially distinct, with alterations to adrenergic constraint on perfusion dominating with regard to the regulation of bulk perfusion, and the endothelial dysfunction playing a stronger role in terms of the higher resolution matching of blood flow distribution to metabolic demand (15). When taken into account with our previous studies demonstrating the potential for increased perfusion heterogeneity at microvascular bifurcations as a defining characteristic of the metabolic syndrome in skeletal muscle of OZR (16), a greater understanding of the impact of the metabolic syndrome on integrated microvascular function represented a critical need. As such, the present studies were designed to determine whether the initial observation of perfusion heterogeneity at arteriolar bifurcations is present throughout microvascular networks, what the contributing mechanisms to this effect are, if they are spatially distinct, and what the functional outcomes of the successive perfusion distribution events would be for blood flow at the level of the terminal arterioles.
The foremost observation from the present study was that the increased heterogeneity at microvascular bifurcations within skeletal muscle (γ) of OZR was identified over four distinct bifurcation classes spanning five arteriolar diameter ranges. While γ at bifurcations in LZR tended to be slightly lower than 0.54 across all levels of arteriolar/bifurcation ranges, this value was elevated toward 0.60 in OZR. The axis limits on the ordinates in Figs. 2–5 were chosen to reflect the observed range of γ. While on the absolute scale, the observed differences in γ are relatively small, these differences are significant statistically, and their cumulative effects translate into physiologically significant differences in function. The consistency of the elevated γ within the microcirculation of OZR afflicted with the metabolic syndrome suggests that this increased heterogeneity of perfusion at bifurcations may represent a defining characteristic of PVD in this animal model. This observation suggests that, as blood flow enters into the microvascular networks of the skeletal muscle of OZR, there is an increased heterogeneity of distribution at bifurcations involving the larger-resistance arterioles, where the result is the generation of “high flow” and “low flow” daughter arterioles arising at this bifurcation. Essentially, this represents a source for increased variability compared with perfusion distribution in LZR, and through a repetitive iteration at successive bifurcations will result in a striking increase to the heterogeneity of arteriolar perfusion within the distal microcirculation; predicted in Fig. 6.
Our recent study that initially identified the presence of an altered γ in the skeletal muscle microcirculation of OZR suggested that this effect was a function of combined alterations to adrenergic tone and the evolving endothelial dysfunction in the metabolic syndrome, as acute interventions against these negative outcomes changed γ to levels that were similar to those determined in LZR (16). Although the present results provide compelling evidence of the consistency of the alteration to γ throughout a microvascular network within OZR skeletal muscle, data from these experiments also demonstrated a significant heterogeneity in terms of the physiological/cellular mechanisms that contribute to the establishment of γ in the metabolic syndrome. In the proximal resistance arterioles, spanning 1A and 2A arterioles, the alteration in γ in OZR was strongly a function of alterations to adrenergic behavior at the microvessel, as treatment with the adrenoreceptor antagonist almost completely removed the change in this parameter and resulted in γ of ∼0.5. What was particularly striking in the present results was that acute interventions designed to ameliorate microvascular dysfunction, the antioxidant TEMPOL, and the PGH2/TxA2 receptor antagonist SQ-29548, had no discernible impact on γ at the level of the 1A and 2A arteriolar bifurcations and only were associated with an improved γ under conditions where adrenoreceptor blockade was also evident. This relationship gradually changed with longitudinal position in the microvascular networks, such that in smaller-diameter bifurcations (2A–3A and 3A–4A), the impact of phentolamine on improving γ at bifurcations was progressively diminished, while the effects of TEMPOL and SQ-29548 on restoring γ to levels identified in LZR was increased. This effect continued to the most distal levels of the microcirculation (4A–5A), where adrenoreceptor blockade was entirely without significant effect and restoring endothelial function resulted in a maximal recovery in γ. In support of our previous initial observations, combined treatment with an adrenoreceptor antagonist and either an antioxidant or the PGH2/TxA2 receptor blocker restores γ to levels determined in LZR throughout the microvascular network. With the incorporation of the results from the present study, the amelioration of different contributing mechanisms of dysfunction, while varying in proportion across levels of the microcirculation, suggests that a multifaceted approach against the microvasculopathy in OZR is required to significantly improve integrated perfusion outcomes.
One of the more intriguing results of the present study is the lack of demonstrated evidence for a role for vascular nitric oxide bioavailability in the establishment of γ in OZR. The observation that treatment of OZR with l-NAME was without effect on γ in microvascular networks in that strain was not surprising, as multiple previous studies have demonstrated that vascular NO bioavailability is dramatically reduced in OZR at this age and treatment and that further antagonism of nitric oxide synthase is without effect (18). However, as previous studies have demonstrated that antioxidant treatment improves vascular NO bioavailability in OZR (16, 17, 22), the lack of an effect on γ following treatment with TEMPOL and l-NAME (where TEMPOL alone improved γ) provides compelling evidence that physiological levels of NO bioavailability may not play a significant role in establishing γ in OZR. The current study employed pharmacological interventions targeted at determining the role of NO bioavailability (l-NAME and TEMPOL) treatments. As such, this study does not rule out the possibility that NO in other “stored” forms, such as nitrosothiols could contribute to changes in vascular function and perfusion distribution, as any roles for these sources for NO were not directly interrogated.
Although there is considerable ongoing effort to identify the molecular signaling cascades that underlying specific identified sites of vascular dysfunction in the metabolic syndrome (2, 29, 30, 32), these efforts can suffer from a lack of contextual relevance, as it becomes exceptionally difficult to integrate these myriad observations from highly reduced preparations into either a functioning tissue type (e.g., vascular smooth muscle cell, isolated microvessel) or into an integrated system (e.g., blood-perfused skeletal muscle). The present study incorporates individual levels of γ into a predictive outcome for tissue perfusion distribution in the distal microcirculation. Using a dichotomous branching network of eight successive bifurcations and the determined levels of γ from the present study, we are able to predict the perfusion distribution in a network consisting of 256 terminal arterioles (5A). In these initial simulations, the resulting frequency distributions reveal that distal arteriolar perfusion in OZR was substantially more heterogeneous than in LZR, with a distribution characterized by an increased interquartile difference that reflects the genesis of a large number of ischemic pathways and a small number of very high flow pathways that may represent functional thoroughfares. While adrenoreceptor blockade and improving endothelial dysfunction with TEMPOL or SQ-29548 each resulted in some improvement to the perfusion distribution characteristics in the distal microcirculation, only combined interventions with both phentolamine and TEMPOL/SQ-29548 allowed for a maximal improvement (i.e., recovery) in the frequency distribution and its descriptive characteristics (Table 3) toward levels predicted in LZR. Obviously, distal arteriolar perfusion distribution within the skeletal muscle will be neither discrete nor clustered, and this is an artifact of the simulation employed. However, our simulation does illustrate both a real broadening of the distribution with the presence of PVD in OZR, and the capacity for ameliorating these negative outcomes with appropriately targeted interventions.
Although these predicted perfusion distributions are based on observation of γ at individual microvascular bifurcations in resting muscle, they may provide a partial explanation for the results from our recent study that evaluated the fatigue resistance of in situ skeletal muscle of OZR across levels of elevated metabolic demand (15). In that study, while the gastrocnemius muscle fatigue rate was elevated in OZR compared with that in LZR, single pharmacological interventions were of extremely limited effectiveness in improving the ultimate functional outcome of increased muscle performance. While adrenoreceptor blockade with phentolamine improved bulk perfusion and active hyperemia, improvements to muscle oxygen extraction, uptake (V̇o2) and oxygen conductance (DmO2) were minimal. Further, improving endothelial function with TEMPOL or SQ-29548 tended to improve extraction, V̇o2 and DmO2, although minimal impacts on bulk perfusion and muscle performance were identified. A significant improvement in muscle fatigue resistance was identified only when combined interventions with phentolamine and TEMPOL/SQ-29548 were applied, and this was also associated with the improvements to bulk perfusion, O2 extraction, V̇o2 and DmO2 (15, please see Ref. 37 for a full review). When combined with the results of the present study, these data strongly suggest that the multifaceted approach to reducing proximal resistance to bulk perfusion in combination with improving distal microvascular perfusion-demand matching represent a requisite condition to lessen the impact of PVD on muscle performance outcomes.
One of the main considerations that must be addressed in the present study is that of the level of resolution for the data. The in situ cremaster muscle preparation allows for very high resolution measurements of multiple arterioles throughout the tissue for both their mechanical (dimension) and hemodynamic (RBC flux, volume blood flow) status. However, this preparation does not lend itself well to the integrated study of the entire network at one time. We have employed the in situ gastrocnemius muscle preparation for the integrated assessment of network function using tracer washout kinetics, as the anatomy and the range of blood flow volumes to the muscle allow for an effective tracer washout protocol that is not possible in the cremaster. Although the strong associations between γ in cremaster muscle, its physiological mechanistic contributors, and its distribution with the results in the in situ gastrocnemius muscle in our previous study is compelling, the intravascular washout experiments summarized in Figs. 8 and 9 provide independent data that reflect the flow distributions throughout the gastrocnemius muscle circulation. Since the washout of 125I-labeled albumin (which is confined to the intravascular space) introduced into the femoral artery immediately proximal to the gastrocnemius muscle reflects the whole-network flow distribution, any potential for a selection bias is eliminated. As presented in Figs. 7 and 8, striking differences in the washout kinetics were determined between LZR and OZR under the conditions of the present study. In general, OZR manifested an earlier appearance time of tracer than in LZR, as well as a longer retention in the microcirculation. Speculatively, this change could reflect the combined influence of both the “high-flow” and “low-flow” pathways that are demonstrated at bifurcations, and it is consistent with the frequency distributions of predicted distal arteriolar perfusion summarized in Fig. 6. While treatment of OZR with phentolamine, TEMPOL, or SQ-29548 all exhibited the ability to partially restore the washout kinetics to levels determined in LZR, combined therapy, which would result in an improved microvascular function and γ across all levels of the networks, resulted in the greatest restoration of the tracer washout kinetics. One particularly intriguing element of the present study was also revealed through the use of the tracer washout approach. Given that the mean transit times for tracer washout was at least 15 s under all conditions, despite arteriolar perfusion velocities that would suggest a more rapid tracer appearance, this suggests that a significant dispersion of the tracer may have also occurred across the capillary beds and within the postcapillary venular networks. While alterations to venular function have been unexplored in this model, interrogating how alterations to venular reactivity and hemodynamics can impact perfusion responses may represent an exciting area for future investigation. The reader is directed to our computational modeling manuscript (39), where these tracer washout kinetics are analyzed using a computational model for microvascular perfusion distributions in OZR vs. LZR skeletal muscle, revealing additional insights into network flow distributions associated with these washout data.
The description of a novel perfusion distribution parameter, γ, alterations to which contributes to a substantially increased heterogeneity in blood flow distribution within the distal microcirculation of the skeletal muscle of OZR, is strikingly relevant to the concept of the “network Fahraeus effect” described by Pries et al. in the late 1980s (33). As a brief summary, the nonuniform distribution of perfusion at successive bifurcations within a microvascular network, contributes to a reduction in the mean microvascular hematocrit (HMV) measured within the distal microcirculation and capillaries (12, 28). This process is distinct from the classically described vessel Fahraeus effect, wherein the differential mean velocities of plasma and erythrocytes within individual microvessels causes a reduction in the instantaneous “tube” hematocrit within a microvessel (4, 27). However, the combined influence of the normal contributing mechanisms to hematocrit reduction within the microcirculation and the effects of an increased γ or accentuated network Fahraeus effect could lead to a reduction from normal HMV within OZR vs. LZR that could contribute to the impaired muscle fatigue resistance demonstrated in our previous studies (15, 20). It is important to state that the results from present study do not account for the effects of plasma skimming at bifurcations, inherent within the network Fahraeus effect (33), on our measurements of arteriolar blood flow and that this process may have impacted the washout kinetics of 125I-labeled albumin in an uncontrolled fashion (as this is an intravascular tracer). Bearing this in mind, further studies will be required to interrogate the speculative argument outlined above, as well as the potential impact of targeted interventions in improving HMV in OZR.
In keeping with this concept, the results from a several earlier studies resulted in a competing argument regarding the degree of heterogeneity in capillary perfusion. While results from Tyml's group suggested that capillary perfusion heterogeneity was reduced with elevated metabolic demand and the inherent vasodilation, suggesting a significant, yet unidentified, control mechanism (36), results from Duling's group did not support this concept, suggesting that capillary perfusion heterogeneity was not a controlled variable in skeletal muscle (10). The results of the present study may be conservatively interpreted as supporting the former argument, where treatment with phentolamine in OZR increased flow and narrowed the perfusion frequency distribution (Table 3). Further, as an improvement to endothelial function in OZR following treatment with TEMPOL and/or SQ-29548 also decreased perfusion heterogeneity, this also suggests that the normal vascular and endothelial function may contribute to the regulation of capillary perfusion heterogeneity. However, as these previous studies (10, 36) used a metabolic stimulus as the primary intervention, while the current study was conducted in resting muscle, additional experiments must be performed to provide a stronger argument regarding the degree of control over capillary perfusion heterogeneity.
Perspectives and Significance
The results of the present study provide for a new conceptual framework with regard to the study of PVD in the metabolic syndrome. Foremost, these results highlight the critical importance of integration across levels of resolution in efforts to interrogate complex disease states. While previous work has identified multiple signaling pathways and elements that may contribute to specific indices of vascular dysfunction (2, 29, 30, 32), there has been a limited effort to determine how these actually impact perfusion responses in specific vessels (16, 41) and almost no study of how these integrate to produce an impaired perfusion response across an entire network. A description of network flow heterogeneity introduced here, with values of the bifurcation ratio γ determined as a function of arteriolar generation, can help to reveal specific mechanistic contributors to PVD, including alterations to adrenergic constriction, vascular oxidant stress, and increased actions of TxA2. This new conceptual framework is strongly predictive of the most translationally relevant outcomes associated with amelioration of PVD, and it will be useful in designing and interpreting future studies of PVD in the metabolic syndrome.
GRANTS
This study was supported by grants from the National Institutes of Health (NIH DK R01 64668 and RR 2865AR) and the American Heart Association (AHA SDG 0330194N and EIA 0740129N).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors also wish to express their gratitude for the expert technical assistance, support and insight from Dr. Julian H. Lombard and Mr. Tianjian Huang from the Department of Physiology at the Medical College of Wisconsin, Ms. Milinda James from the Department of Physiology and Pharmacology, and Dr. Stephanie J. Frisbee from the Department of Community Medicine at West Virginia University and for support provided through the Translational Research Facility in the Center for Cardiovascular and Respiratory Sciences at the West Virginia University Health Science Center.
REFERENCES
- 1. Aboyans V, Lacroix P, Criqui MH. Large and small vessels atherosclerosis: similarities and differences. Prog Cardiovasc Dis 50: 112–125, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Ali MI, Ketsawatsomkron P, Belin de Chantemele EJ, Mintz JD, Muta K, Salet C, Black SM, Tremblay ML, Fulton DJ, Marrero MB, Stepp DW. Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone. Circ Res 105: 1013–1022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker M, Wayland H. On-line volume-flow rates and velocity profile measurements for blood in microvessels. Microvasc Res 7: 131–143, 1974 [DOI] [PubMed] [Google Scholar]
- 4. Barbee JH, Cokelet GR. The Fahraeus effect. Microvasc Res 3: 6–16, 1971 [DOI] [PubMed] [Google Scholar]
- 5. Bassingthwaighte JB, Liebovitch LS, West BJ. Intraorgan flow heterogeneities. In: Fractal Physiology. New York: Oxford University Press, 1994, p. 236–262 [Google Scholar]
- 6. Bassingthwaighte JB, Goresky CA. Modeling in the analysis of solute and water exchange in the microvasculature. In: Handbook of Physiology. The Cardiovascular System. Microcirculation. Bethesda, MD: Am. Physiol. Soc., 1984, sect. 2, vol. IV, pt. 1, chapt. 13, p. 549–626 [Google Scholar]
- 7. Bohlen HG. Protein kinase betaII in Zucker obese rats compromises oxygen and flow-mediated regulation of nitric oxide formation. Am J Physiol Heart Circ Physiol 286: H492–H497, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Bouvet C, Belin de Chantemèle E, Guihot AL, Vessières E, Bocquet A, Dumont O, Jardel A, Loufrani L, Moreau P, Henrion D. Flow-induced remodeling in resistance arteries from obese Zucker rats is associated with endothelial dysfunction. Hypertension 50: 248–254, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Cooke JP, Wilson AM. Biomarkers of peripheral arterial disease. J Am Coll Cardiol 55: 2017–2023, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damon DH, Duling BR. Evidence that capillary perfusion heterogeneity is not controlled in striated muscle. Am J Physiol Heart Circ Physiol 249: H386–H392, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Davis MJ. Determination of volumetric flow in capillary tubes using an optical Doppler velocimeter. Microvasc Res 34: 223–230, 1987 [DOI] [PubMed] [Google Scholar]
- 12. Desjardins C, Duling BR. Microvessel hematocrit: measurement and implications for capillary oxygen transport. Am J Physiol Heart Circ Physiol 252: H494–H503, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Dunbar JA, Reddy P, Davis-Lameloise N, Philpot B, Laatikainen T, Kilkkinen A, Bunker SJ, Best JD, Vartiainen E, Kai Lo S, Janus ED. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care 31: 2368–2373, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ford ES, Mokdad AH. Epidemiology of obesity in the Western Hemisphere. J Clin Endocrinol Metab 93: S1–S8, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Frisbee JC, Butcher JT, Goodwill AG, Olfert IM. Divergence between arterial perfusion and fatigue resistance in skeletal muscle in the metabolic syndrome. Exp Physiol 96: 369–383 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frisbee JC, Hollander JM, Brock RW, Yu HG, Boegehold MA. Integration of skeletal muscle resistance arteriolar reactivity for perfusion responses in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 296: R1771–R1782, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frisbee JC, Delp MD. Vascular function in the metabolic syndrome and the effects on skeletal muscle perfusion: lessons from the obese Zucker rat. Essays Biochem 42: 145–161, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Frisbee JC. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 289: R307–R316, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Frisbee JC. Enhanced arteriolar alpha-adrenergic constriction impairs dilator responses and skeletal muscle perfusion in obese Zucker rats. J Appl Physiol 97: 764–772, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Frisbee JC. Impaired skeletal muscle perfusion in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 285: R1124–R1134, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Frisbee JC, Maier KG, Stepp DW. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol 283: H2160–H2168, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Fulton D, Harris MB, Kemp BE, Venema RC, Marrero MB, Stepp DW. Insulin resistance does not diminish eNOS expression, phosphorylation or binding to HSP-90. Am J Physiol Heart Circ Physiol 287: H2384–H2393, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Goodwill AG, James ME, Frisbee JC. Increased vascular thromboxane generation impairs dilation of skeletal muscle arterioles of obese Zucker rats with reduced oxygen tension. Am J Physiol Heart Circ Physiol 295: H1522–H1528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamilton WF, Moore JW, Kinsman JM, Spurling RG. Studies on the circulation IV. Further analysis of the injection method, and of changes in hemodynamics under physiological and pathological conditions. Am J Physiol 99: 534–551, 1932 [Google Scholar]
- 25. Johnson FK, Johnson RA, Durante W, Jackson KE, Stevenson BK, Peyton KJ. Metabolic syndrome increases endogenous carbon monoxide production to promote hypertension and endothelial dysfunction in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 290: R601–R608, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Kannan H, Thompson S, Bolge SC. Economic and humanistic outcomes associated with comorbid type-2 diabetes, high cholesterol, and hypertension among individuals who are overweight or obese. J Occup Environ Med 50: 542–549, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Klitzman B, Duling BR. Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol Heart Circ Physiol 237: H481–H490, 1979 [DOI] [PubMed] [Google Scholar]
- 28. Keller MW, Damon DN, Duling BR. Determination of capillary tube hematocrit during arteriolar microperfusion. Am J Physiol Heart Circ Physiol 266: H2229–H2238, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Ma L, Ma S, He H, Yang D, Chen X, Luo Z, Liu D, Zhu Z. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res 33: 446–453, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 54: 1384–1392, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Naik JS, Xiang L, Hester RL. Enhanced role for RhoA-associated kinase in adrenergic-mediated vasoconstriction in gracilis arteries from obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 290: R154–R161, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol 30: 1711–1717, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pries AR, Ley K, Gaehtgens P. Generalization of the Fahraeus principle for microvessel networks. Am J Physiol Heart Circ Physiol 251: H1324–H1332, 1986 [DOI] [PubMed] [Google Scholar]
- 34. Rattigan S, Wheatley C, Richards SM, Barrett EJ, Clark MG. Exercise and insulin-mediated capillary recruitment in muscle. Exerc Sport Sci Rev 33: 43–48, 2005 [PubMed] [Google Scholar]
- 35. Stepp DW, Frisbee JC. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol 282: H816–H820, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Tyml K, Cheng L. Heterogeneity of red blood cell velocity in skeletal muscle decreases with increased flow. Microcirculation 2: 181–193, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Vessieres E, Belin de Chantemèle E, Toutain B, Guihot AL, Jardel Loufrani A, L, Henrion D. Cyclooxygenase-2 inhibition restored endothelium-mediated relaxation in old obese Zucker rat mesenteric arteries [Online]. Front Vasc Physiol 1: 2010. www.frontiersin.org/Journal/Abstract.aspx?s=1141&name=vascularphysiology&ART_DOI=10.3389/fphys.2010.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wagner PD. Gas exchange and peripheral diffusion limitation. Med Sci Sports Exerc 24: 54–58, 1992 [PubMed] [Google Scholar]
- 39. Wu F, Beard DA, Frisbee JC. Computational analyses of intravascular tracer washout reveal altered capillary-level flow distributions in obese Zucker rats. J Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol 294: H1658–H1666, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Xiang L, Naik JS, Hodnett BL, Hester RL. Altered arachidonic acid metabolism impairs functional vasodilation in metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 290: R134–R138, 2006 [DOI] [PubMed] [Google Scholar]