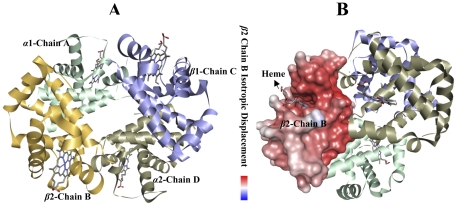
Figure 5. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution.
The haems are located toward the proximal region; the partition between the mean planes of N (porphyrin) and C (porphyrin) being 0.16(6) A and 0.10(6) A, respectively at the alpha and beta haems. At the alpha haems, the normal's to the average pyrrole planes are skewed evenly toward the haem centre, by about three degrees relative to the haem normal, and there is a folding of about four degrees of the haem about an axis running between the methene carbons that are between the pyrrole rings bearing like-type side-chains. At the beta haems, there is no such folding (Figure 5a) having α1-Chain A (pale green), β2-Chain B (yellow), β1-Chain C (violet) and α2-Chain D (grey) which has four haems in each chain but this study was focused on β2-Chain B similar to the isotropic displacement model (Figure 5b).