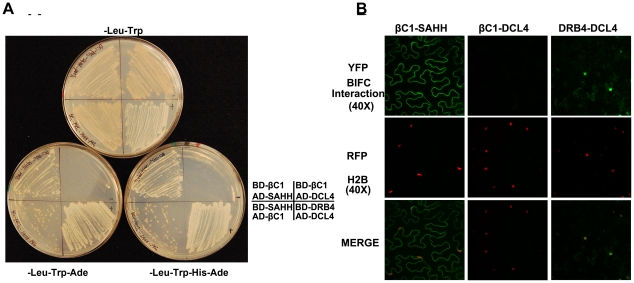
Figure 6. βC1 protein interacts with SAHH.
(A) Yeast two-hybrid interaction. Bait proteins were expressed as GAL4 DNA binding domain fusions, and prey proteins as GAL4 activation domain fusions, in yeast PJ649A cells. Growth on media lacking leucine (-Leu) and tryptophan (-Trp) indicates maintenance of the bait and prey plasmids. Interaction is indicated by growth on plates also lacking adenine (-Ade), or histidine and adenine (-His-Ade). (B) BiFC interaction. Constructs expressing βC1 and SAHH fused to the N- or C-terminal portions of YFP were delivered to N. benthamiana leaf cells by agroinfiltration. Cells were photographed 48 hours post-infiltration at 40 X magnification using a confocal laser scanning microscope. RFP-histone 2B (RFP-H2B) was used as a marker for the nucleus. The co-expressed proteins are indicated above the photographs, which are representative of results with all possible combinations of fusion proteins. In both yeast two-hybrid and BiFC experiments, DRB4 was co-expressed with DCL4 as a positive control, and βC1 was expressed with DCL4 as a negative control.