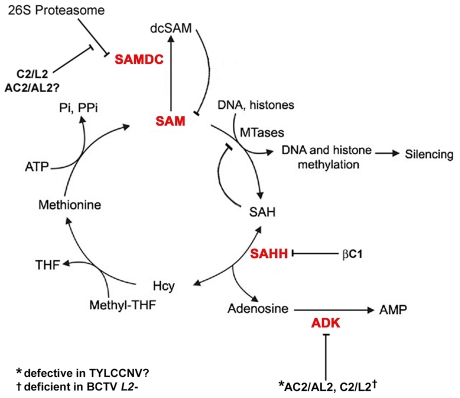
Figure 9. Model for methyl cycle inactivation by geminivirus and betasatellite proteins.
S-adenosyl methionine (SAM) is the methyl donor for most transmethylation reactions. The product, S-adenosyl homocysteine (SAH), inhibits transmethylation by competing with SAM for methyltransferases (MTases). SAH is converted to homocysteine (Hcy) and adenosine by S-adenosyl homocysteine hydrolase (SAHH). Phosphorylation of adenosine by adenosine kinase (ADK) is critical because the SAHH-catalyzed reaction is reversible and the equilibrium lies in the direction of SAH synthesis. By removing adenosine, ADK promotes flux through the cycle and SAM production, and minimizes competitive inhibition of methyltransferase reactions by SAH. Thus, ADK inactivation by geminivirus AC2/AL2 and C2/L2 proteins globally interferes with methylation. Data presented in this report suggests that TYLCCNV AC2/AL2 may be deficient for ADK inhibition. The C2/L2 protein has also been shown to stabilize SAM decarboxylase (SAMDC), which causes decarboxylated SAM (dcSAM) levels to rise. dcSAM is a competitive inhibitor of SAM. Whether AC2/AL2 proteins also promote increases in dcSAM has yet to be tested. Data in this report indicates that the betasatellite encoded βC1 protein directly antagonizes the methyl cycle by inhibiting SAHH. Note that this diagram lists only DNA and histones as methyltransferase substrates, although any type of transmethylation reaction may require SAM as a cofactor. THF: tetrahydrofolate, PPi: pyrophosphate; Pi: inorganic phosphate.