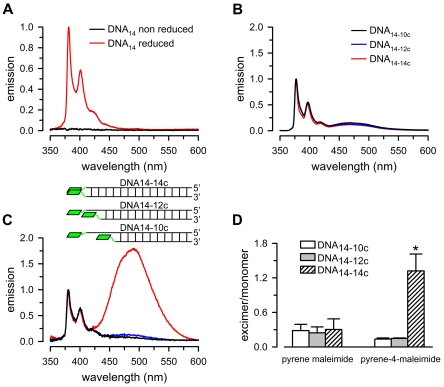
Figure 3. Emission of pyrene compounds reacted with thiol-modified DNA.
A. Emission spectra of single-stranded DNA containing a 5′ end thiol group (DNA14) reacted with pyrene-4-maleimide. Data were normalized to peak 1 intensity from reduced DNA14. [DNA14] was 0.5 µM, and [pyrene-4-maleimide] was 2 µM. B. Emission spectra of double-stranded DNA reacted with pyrene maleimide. DNA14-14c: DNA14 annealed to fully complementary DNA with a 3′ end thiol group (DNA14c). DNA14-12c: DNA14 annealed to a 3′ end two-base shorter complementary oligonucleotide with a 3′ end thiol group. DNA14-10c: DNA14 annealed to a 3′ end four-base shorter complementary oligonucleotide with a 3′ end thiol group. C. Emission spectra of double-stranded DNA reacted with pyrene-4-maleimide. Insert: schematic representation of the experimental system showing the double-stranded DNAs labeled with pyrene-4-maleimide. The pyrenes are represented by green rhomboids. Data in panels B and C were normalized to peak 1 intensity. The labels in panel B also apply to panel C. D. Excimer/monomer emission ratio. The values were calculated as: excimer/monomer = Ipeak 4/Ipeak 1, where I is the highest intensity of the peak, and peak 1 and peak 4 correspond to the excimer and monomer emission peaks. Averages ± SEM from experiments such as those shown in panels B and C (n = 3 for pyrene maleimide, and n = 4 for pyrene-4-maleimide). The asterisk denotes P<0.05 for the pyrene-4-maleimide DNA14-14c adduct vs. each of the other adducts presented in panel D. The concentrations of the fluorescent probes and double-stranded DNA were 4 µM and 0.5 µM, respectively.