Abstract
Exercise improves performance on a number of hippocampus involved cognitive tasks including contextual fear conditioning, but whether exercise enhances contextual fear when the retention interval is longer than 1 day is not known. Also unknown is whether exercise improves trace conditioning, a task that requires the hippocampus to bridge the time interval between stimuli. Hence, 4-month-old male C57BL/6J mice were housed with or without running wheels. To assess whether hippocampal neurogenesis was associated with behavioral outcomes, during the initial ten days, mice received Bromodeoxyuridine to label dividing cells. After 30 days, one group of mice was trained in a contextual fear conditioning task. Freezing to context was assessed 1, 7, or 21 days post-training. A separate group was trained on a trace procedure, in which a tone and footshock were separated by a 15, 30, or 45 sec interval. Freezing to the tone was measured 24 hrs later in a novel environment, and freezing to training context was measured 48 hrs later. Running enhanced freezing to context when the retention interval was 1, but not 7 or 21 days. Running had no effect on trace conditioning even though runners displayed enhanced freezing to the training context 48 hrs later. Wheel running increased survival of new neurons in the hippocampus. Collectively, findings indicate that wheel running enhances cognitive performance on some tasks but not others and that enhanced neurogenesis is not always associated with improved performance on hippocampus tasks, one example of which is trace conditioning.
Keywords: Exercise, learning, retention interval, hippocampus, neurogenesis
INTRODUCTION
Aerobic exercise has beneficial effects on cognitive performance. In humans, regular exercise has been associated with enhanced performance on tasks of executive function, working memory, and spatial memory [1–3]. In agreement, animal studies consistently show enhanced performance in tests of associative and spatial learning in animals given access to running wheels [4–8]. Many of these enhancements are observed in behavioral tasks that rely on the hippocampus. For instance, wheel running improves performance in the water maze and enhances contextual fear conditioning relative to sedentary animals [5, 8, 9]. Clearly engaging in exercise has beneficial effects though further work is needed to elucidate the specific cognitive processes enhanced by exercise.
Induction of conditioned fear leads to the development of enduring fear memories that persist for 30 and even 60 days after training [10, 11]. Evidence indicates that different brain regions participate in the expression of conditioned fear as the retention interval increases. The hippocampus plays a role in the initial acquisition and consolidation of contextual fear memories [12, 13]. Neocortical areas appear to mediate recall of fear memories as the time between training and testing increases [10, 12]. To date research has focused on the ability of exercise to enhance acquisition of contextual fear conditioning when testing occurs shortly after training [5, 6, 8]. Given that different brain regions mediate the recall of fear memories depending on the time between training and testing exercise may have different effects on behavior depending on the retention interval. Currently unknown is whether the beneficial effects of exercise change as a function of the retention interval. If the pro-cognitive effects of exercise are mainly the result of changes taking place in the hippocampus one possibility is that wheel running may have time-dependent effects on performance as information is transferred from the hippocampus to cortical regions. Additionally prior work has indicated that running improves memory consolidation suggesting that running has its effects during the initial stages of memory formation [7, 8]. Examining behavior across varying retention intervals may further our understanding of how exercise facilitates memory formation.
The hippocampus participates in many cognitive processes including spatial navigation, associative learning, and associating temporally discontiguous stimuli [14–19]. When two stimuli or events are separated by time, the hippocampus is proposed to provide an associative link to span the time between the stimuli presentation in order for an association to form. Evidence that the hippocampus is critical for associating stimuli separated in time comes from experiments that employ a trace conditioning task. In contrast to the commonly used delay conditioning procedure, where presentation of the conditioned stimulus (typically a tone; CS) and the unconditioned stimulus (typically a mild footshock; US) overlap, in the trace procedure there is a gap between the CS and US presentation. Trace conditioning, is generally considered a more challenging task than delay conditioning as animals take longer to acquire trace conditioning [18]. Acquisition of trace conditioning appears to be dependent on the hippocampus, as studies have consistently shown deficits following lesions or pharmacological inactivation of the hippocampus [20, 21]. In contrast, delay conditioning is acquired independent of the hippocampus. Prior work has shown that manipulations that disrupt context fear learning often impair performance in a trace conditioning task [21]. Given the role of the hippocampus in trace conditioning one possibility is that treatments associated with improved hippocampal function (e.g., wheel running or environmental enrichment) may facilitate performance in a trace conditioning task.
Exercise induces a host of changes within the central nervous system (CNS), many of which occur in the hippocampus. For instance, exercise increases production of trophic factors that promote growth and repair, blood flow, long-term potentiation, synaptic plasticity, and adulthood hippocampal neurogenesis [4, 5, 8, 22–24]. Evidence indicates the exercise-induced increase in hippocampal neurogenesis may mediate some of the beneficial effects of exercise on learning and memory. For example, reducing neurogenesis via irradiation was found to prevent the exercise-induced enhancement in spatial learning [5]. Studies evaluating whether hippocampal neurogenesis is important for acquiring contextual fear have shown mixed results. Our laboratory and others found no deficit in contextual conditioning when neurogenesis is reduced [5, 25] whereas others report impairments [26–28]. These differences in findings may relate to the testing procedure as Drew et al. [29] found that reducing hippocampal neurogenesis disrupted contextual fear conditioning when training consisted of a single, but not multiple, trial(s). However, prior work indicates that basal levels of hippocampal neurogenesis are important for learning trace conditioning, as inhibiting hippocampal neurogenesis has been found to impair acquisition [25, 30]. Whether an increase in neurogenesis facilitates performance in a trace conditioning task has yet to be determined.
The current study evaluated whether voluntary wheel running enhanced performance in both a contextual and trace conditioning procedure and whether the beneficial effects of exercise depend on the time interval between training and testing. We used a one trial trace conditioning procedure to make the task challenging to learn, as comparing exercise and sedentary mice in other behavioral tasks has shown that if the task is made easier by increase the number of training trials no differences are observed between exercise and sedentary mice [9]. To ensure mice did not show ceiling levels of freeze behavior, that could mask differences between runners and sedentary mice, we opted for the single trial procedure. Further, in a contextual conditioning task hippocampal neurogenesis may only be involved when only a single context-shock pairing is presented whereas increasing the number of pairing diminishes the role of new neurons [29]. Lastly, we assessed the survival of hippocampal neurons to determine whether they were associated with the behavioral changes.
2. METHODS
2.1. Experimental subjects
Subjects were 117 male C57BL/6J mice from The Jackson Laboratory (approx 4 weeks old on arrival). Seventy-two mice were used in Experiment 1 and 45 in Experiment 2. Mice were allowed to acclimate to the animal facility for 2 weeks prior to the start of the experiments. Mice were given ad libitum access to food and water and housed under a 12 hr reverse light/dark cycle in the AAALAC approved animal facility. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals and the experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign.
2.2. Wheel running
Mice were divided into either the exercise (access to a running wheel) or sedentary condition. Mice in the sedentary condition were individually housed in standard polycarbonate shoebox cages (29 cm L × 19 cm W × 13 cm H). Mice in the exercise condition were individually housed in cages with a 23 cm diameter running wheel (36 cm L × 20 cm W × 14 cm H; Respironics, Bend, OR). Wheel rotations were continuously collected in 1 min intervals via magnetic switches interfaced to a computer using the VitalView software (Respironics, Bend, OR). During the initial 10 days of wheel access all mice received daily intraperitoneal injections of Bromodeoxyuridine (BrdU: 50 mg/kg), a thymidine analogue that incorporates into dividing cells.
2.2.1. Experiment 1: Effect of exercise on contextual fear conditioning
After 30 days of wheel access or sedentary housing, mice were trained on a contextual fear conditioning task. All training/testing occurred during the dark phase of the light cycle (mouse’s active phase). Following the training procedure of Frankland et al. [12], mice were individually placed into a novel square chamber (32 cm L × 28 cm W × 30 cm H, dark grey walls) with a metal bar floor connected to a shock scrambler controlled by a digital timer (Med Associates, St. Albans, VT, USA). Mice were allowed to acclimate and explore the chamber for two minutes. After the acclimation period, half of the mice were presented with five unsignaled footshocks (2 sec, 0.70 mA) spaced 1 minute apart. Control mice in the non-shock condition were individually placed in the same chamber for 7 min, but did not receive any footshocks. Following training, mice were returned to their home cage. Mice were placed back into the training chamber to evaluate memory 1, 7, or 21 days later by measuring freezing to the training context (6 min). Freezing was recorded by TopScan video tracking software (CleverSystems, Reston, VA, USA) as the total number of seconds when the animal’s center of mass did not register horizontal movement (±1 mm). Freezing data were converted into percent time spent freezing by dividing the total number of seconds a mouse spent freezing by the total number of seconds of testing and multiplied by 100. Ninety minutes after testing, all mice were euthanized by transcardial perfusion with 4% paraformaldehyde in phosphate buffer solution for analysis of hippocampal neurogenesis via immunohistochemistry (described below).
2.2.2. Experiment 2: Effects of wheel running on trace fear conditioning
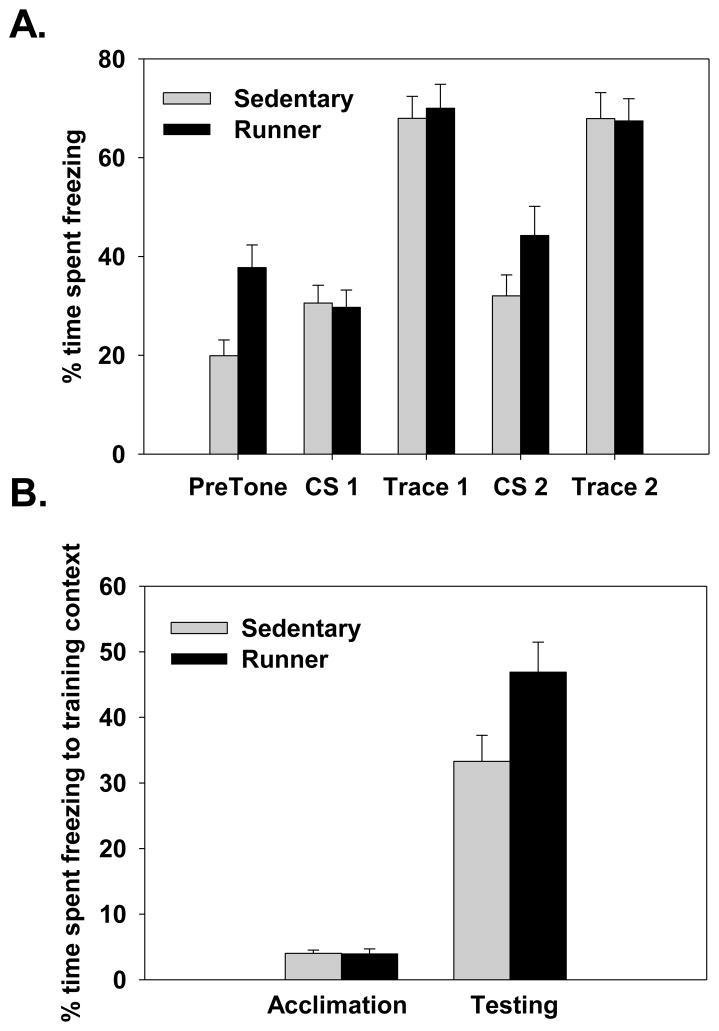
After 30 days of wheel access or sedentary housing, mice were trained on a trace fear conditioning task based on method of Misane et al. [31]. Figure 1 illustrates the trace training procedure. Exercise and sedentary mice were individually transported to a testing room and placed into a dark grey square chamber (same apparatus used in Experiment 1) and allowed to explore for 120 to 150 seconds (see Figure 1). After acclimation, mice were presented with a single tone followed by a single footshock (2 sec, 0.70 mA) after a trace interval of 15, 30, or 45 seconds (see Figure 1). Total time spent in the training chamber was the same for all mice (217 sec). The following day mice were tested for freezing to the CS (i.e., tone) in a novel context. Mice were placed into an octagon shaped chamber with white and black striped walls and a smooth floor. Additionally, the ambient room light was reduced, a different individual handled the mice, and black curtains were hung around the testing room to minimize similarity to the testing environment during training. Mice were placed into the novel environment for a total of 6 minutes. During the first 3 minutes freezing to the novel context (Pre-tone) was measured. After 3 minutes a 30 second CS (tone, 86 dB) was presented (CS 1) followed by a 60 second trace (Trace 1) and then the tone (CS 2) was presented a second time followed by another trace (Trace 2) and freezing was measured as the total number of seconds the animal did not move and was converted into a percentage by dividing the number of seconds when the animal did not move by the total duration of each segment of the testing phase (see Figure 1) and multiplied by 100.
Figure 1.
Trace conditioning procedure employed in Experiment 2. First three boxes show the timing of CS and US presentations at the three trace intervals used during training. The fourth box shows the auditory fear testing procedure conducted in a novel context 24 hrs after training.
Twenty-four hours after assessing freezing to the tone, mice were placed back in the original training chamber, but no tone was presented (4 minutes). As in Experiment 1, freezing was recorded by video tracking software and measured as the total number of seconds the mouse did not register horizontal movement.
2.3. Immunohistochemistry
Following perfusions, brains of mice in Experiment 1 (contextual conditioning) were fixed overnight in 4% paraformaldehyde and then transferred into 30% sucrose solution. Brains were sectioned at 40 micrometers using the cryostat. A one-in-six series was stained for BrdU to identify newly divided cells. Briefly, free floating sections were rinsed in tissue buffering solution (TBS) and then treated with 0.6% hydrogen peroxide for 30 min. To denature DNA, sections were placed in a solution of 50% de-ionized formamide and 10% 20x SCC buffer for 120 min at 65 °C. Followed by 10% 20x SCC buffer for 15 min, then 2 N hydrochloric acid for 30 min at 37 °C, then 0.1 M boric acid (pH 8.5) for 10 min. Sections were blocked with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus) for 30 min, and then incubated with primary antibody rat anti-BrdU (1:200; AbD Serotec, Raleigh, NC, USA) at a dilution of 1:200 in TBS-X plus for 72 h at 4 °C. After washing with TBS, sections were treated with TBS-X plus for 30 min and then incubated with a biotinylated goat anti-rat secondary antibody (1:250) in TBS-X plus for 100 min at room temperature. Sections were then treated with the ABC system (Vector, Burlingame, CA, USA) and stained using a diaminobenzidine kit (DAB; Sigma, St. Louis, MO, USA).
To measure the proportion of new cells that differentiation into new neurons a separate one-in-six series was labeled with BrdU and NeuN (mature neuron marker). Free-floating sections were handled as described above with the exception of the use of a cocktail of primary antibodies: rat anti-BrdU (1:200) and mouse anti-neuronal nuclear protein (NeuN; 1:50; Millipore, Billerica, MA, USA). Fluorescent markers (Cy2, Cy3, and Cy5) were conjugated to secondary antibodies, made in goat, at dilution of 1:200 and also delivered as a cocktail, anti-rat, anti-mouse, anti-rabbit. ABC and DAB steps were omitted.
2.4. Image analysis
2.4.1. BrdU positive cells (DAB)
The entire granule layer (bilateral), from a one-in-six series, was imaged by systematically advancing the field of view of a Zeiss brightfield light microscope, and taking multiple photographs, via axiocam interfaced to computer, under 10x (total 100x) magnification. Images were analyzed by ImageJ software. For each image, the granule layer was outlined, and BrdU-positive nuclei were automatically counted by setting a fixed threshold to remove the background. The threshold selected was validated by comparing automated counts to hands counts. In addition, the area (pixels) within the trace was recorded. Estimates of total number of BrdU positive cells are expressed by per cubic micrometer dentate gyrus sampled. Values were further adjusted by removing the fraction of cells predicted to cross the boundary of the section on one side to produce unbiased estimates.
2.4.2. Double labeled BrdU and NeuN positive cells
BrdU-positive cells in the dentate gyrus were micro-analyzed by confocal microscopy to determine whether cells co-expressed NeuN. Number of new neurons per cubic micrometer per mouse was calculated as number of BrdU cells per cubic micrometer (from above) multiplied by average proportion of BrdU cells co-expressing NeuN for a given experimental group.
2.5. Statistical analyses
Distance run (km/day) was analyzed by repeated measures ANOVA with Day as the within-subjects factor. In Experiment 1 (contextual conditioning), duration spent freezing to the context, number of BrdU positive cells, and the number of BrdU cells co-labeled with NeuN were analyzed by 3-way ANOVA with Exercise condition (runner or sedentary), Training procedure (trained or non-shock controls), and Retention interval (1, 7, or 21 days) as the between-subjects factors. In Experiment 2 (Trace conditioning), freezing response to the novel context and CS presentations were analyzed by 3-way repeated measures ANOVA with Time-point (Pre-tone, CS 1, trace 1, CS 2, and trace 2; see Figure 1) as the within-subjects factor and Exercise condition and Trace interval (15, 30, or 45 s) as the between-subjects factors. Duration spent freezing to the context was analyzed by 2-way ANOVA with Exercise condition and Trace interval as the between-subjects factors. p<0.05 was considered statistically significant.
3. RESULTS
3.1. Wheel running data
Distance run varied across the course of Experiment 1 and Experiment 2 (experiment 1 F(50,450)=5.40, p<0.0001; experiment 2 F(43,817)=22.73, p<0.0001; see Figure 2). Running escalated during the initial two weeks of wheel access and then maintained a relatively stable level except during behavior training and testing which occurred on days 30–31 for Experiment 1 (Contextual conditioning) and days 30–32 for Experiment 2 (Trace conditioning). The reduction in wheel running during behavioral testing likely results from the disruption of behavioral testing (removal from the wheels and having researchers entering the room over a six hour period) as prior work from our laboratory has shown that mice will maintain consistent levels of wheel running for more than 50 days if left undisturbed [32] however during behavioral testing running is reduced [5].
Figure 2.
Distance run (km) for mice in Experiment 1 (grey triangles) that were trained on the contextual fear conditioning task and Experiment 2 (black circles) that were trained on the trace conditioning paradigm. Mice in Experiment 1 ran an average distance of 5.73 km/day overall. Mice in Experiment 2 ran an average distance of 6.24 km/day overall. Symbols represent averaged distance run per day ± standard errors of the mean (SEM).
3.2. Experiment 1: Contextual fear conditioning
As expected, non-shock control mice showed minimal freezing behavior compared to mice that underwent the training procedure (F(1,58)=545.06, p<0.0001; see Figure 3). No significant differences were observed between the exercise and sedentary non-shock control mice. The interaction between Exercise condition (runner or sedentary), Training procedure (trained or non-shock control), and Retention interval (1, 7, or 21 days) was significant (F(2,58)=4.07, p<0.05). Post hoc analysis revealed that for mice trained with the footshock, wheel running significantly increased time spent freezing to the context when mice were tested one day after training (p<0.01). However, when freezing to the context was measured 7 and 21 days after training, no difference was observed between the exercise and sedentary mice.
Figure 3.
Percent time spent freezing to the context 1, 7, or 21 days after training. Bars represent mean duration of freezing divided by total test time (6 minutes) and multiplied by 100 to represent freeze time as a percentage.
3.3. Experiment 2: Trace fear conditioning
3.3.1. Freezing to novel context and tone
A repeated measures ANOVA revealed a significant interaction between Exercise condition and Time-point (Pre-tone, CS 1, trace 1, CS 2, and trace 2; see Figure 1) (F(1,39)=10.1, p<0.01; see Figure 4A). Post hoc analysis revealed that during the 3 minutes prior to the tone presentation (PreTone), runners, regardless of the trace interval they were trained with, froze significant more to the novel environment than sedentary mice (p<0.05). No difference in freezing was observed between the runner and sedentary mice after the tone was presented, that is during either CS presentation or during the trace intervals following the CSs. While there was a trend for runners to freeze more during the second CS presentation the difference was not significant (p=0.09ns). A significant main effect of Time-point (F(1,39)=140, p<0.0001) showed that all mice froze more during the trace intervals following the CS presentations compared to the novel context alone.
Figure 4.
(A).Percent time spent freezing to the novel environment prior to tone presentation (PreTone, 3 minutes) and during the different segments of testing after the tone onset (i.e., CS 1– 30 seconds during first CS presentation, Trace 1- 1 min after first CS, CS 2– 30 seconds during second CS presentation, Trace 2- 1 min after second CS). (B). Percent time spent freezing to training (i.e., shock paired) context during the acclimation phase of the training day (i.e., prior to CS-US presentation) and during testing (48 hours after training). Data are collapsed across trace intervals. Bars represent duration of freezing divided by the duration and multiplied by 100 to represent freeze time as a percentage.
3.3.2. Freezing to training context
On the training day mice were allowed to acclimate to the chamber for at least two minutes (see Figure 1). Analysis showed no significant differences in freezing during the acclimation phase (prior to CS and US presentation) during training (F(1,39)=0.034, p>0.05ns; see Figure 4B). Additionally, we found no significant difference in the distance traveled by sedentary or runners during the acclimation phase (F(1,39)=0.92, p>0.05ns) Indicating no baseline differences in freezing behavior or activity levels existed between runners and sedentary mice prior to conditioning.
Forty-eight hours after conditioning runners froze significantly more than sedentary mice to the training context, in the absence of a tone (F(1,39)=5.07, p<0.05; see Figure 4B). No other significant main effects or interactions were found.
3.4 Hippocampal neurogenesis
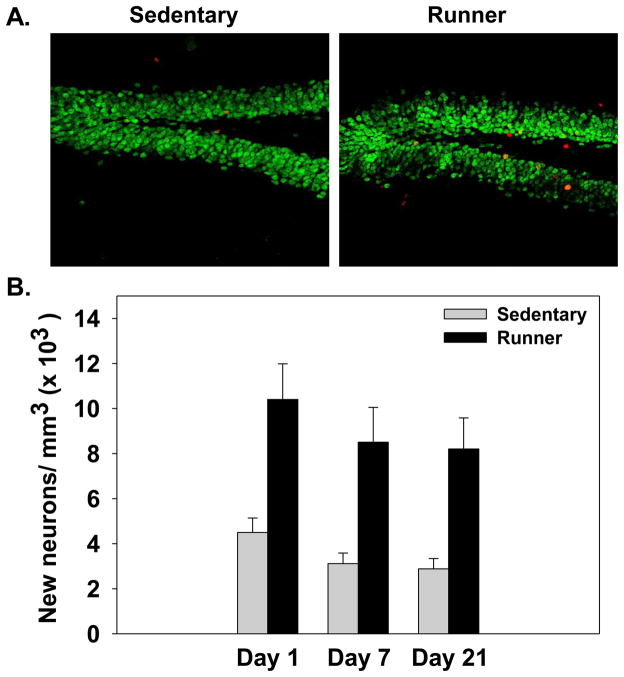
To confirm that running increased neurogenesis in our studies animals from Experiment 1 were analyzed using immunohistochemistry. Running significantly increased the number of BrdU positive cells in the granular cell layer of the hippocampus (F(1,60)=26.32, p<0.0001) compared to sedentary mice. Additionally, exercise enhanced differentiation of new cells into neurons, as wheel running significantly increased the number of BrdU positive cells that co-expressed the neuron marker NeuN (F(1,60)=36.47, p<0.0001). Figure 5 shows the number of BrdU-labeled new neurons in the granular cell layer. No significant effects or interactions with the Training procedure (i.e., trained or non-shock controls) or Retention interval (i.e., 1, 7, or 21 days) were observed.
Figure 5.
(A). Representative coronal sections of the granular cell layer stain for BrdU (red) and NeuN (green) of sedentary and runners from Experiment 1 that were trained on contextual fear conditioning and perfused 21 days after training. (B). Average number of BrdU-labeled new neurons in the granular cell layer of the hippocampus. Bars represent mean number of BrdU positive cells that co-expressed NeuN a mature neuronal marker ± SEM.
4. DISCUSSION
Results extend the existing literature that shows wheel running enhances contextual fear conditioning [5, 6, 8, 33] by demonstrating that this effect depends on the retention interval between training and testing. Additionally, we report that wheel running does not enhance development of the CS-US association when animals are trained on a one trial trace conditioning procedure. These findings argue in favor of selective beneficial effects of wheel running on processing of contextual information that likely facilitate acquisition or consolidation of new information.
Our findings demonstrate that wheel running increased freezing to the context, but only when testing occurred soon after training (i.e., 1 day, but not 7 or 21 day; see Figure 3). The initial advantage in the runners may reflect faster processing and/or the consolidation of the contextual information. Consistent with this interpretation, prior work found that wheel running reduced the time required for an animal to form a representation of a context which the authors propose indicates improved consolidation [8]. Additionally, wheel running has been reported to enhance consolidation of a delay conditioning task [7]. Increasing the retention interval had no effect on runner’s performance, demonstrating that wheel running leads to stable performance regardless of the retention interval. Runners froze at near maximum levels at all of the time-points testing, whereas sedentary mice only showed these high levels of freezing 7 or 21 days after training. Running may still have beneficial effects during the later days of testing, but possibly due to a ceiling level of freezing these benefits cannot be detected in the present task. Whether running leads to prolonged benefits in others behavioral tasks is currently unknown. Overall findings indicate that wheel running enhances contextual learning and that there is a transition point sometime between one of seven days where the sedentary mice catch up.
Alternatively, the improved performance in the sedentary mice and initial enhanced freezing in runners may reflect an increase in nonspecific fear. Balogh et al. [34] reported that C57BL/6J mice show enhanced fear to a shock paired-context when testing occurred 7 to 30 days after training, however, this was accompanied by a parallel increase in freezing to a novel context indicating an increase in generalized fear. The enhanced freezing in sedentary mice at the longer retention intervals may relate to the incubation of fear phenomenon, in which the fear response is strengthened as the time between the initial experience and re-exposure increases [35]. Prior work has shown that running improves contextual discrimination in rats which argues against an exercise-induced increase in generalized fear [8]. However, species differences may exist and future work should assess whether exercise facilitates context discrimination in mice. Wheel running does not enhance fear potentiated startle nor increase reactivity to a footshock in mice [7]. Additionally, we found no differences in baseline locomotor activity between runner and sedentary mice prior to conditioning, indicating that running does not alter non-learning performance factors that could affect their behavior. However, in the current study we found that runners showed enhanced freezing to the novel context prior to the tone presentation (Figure 4A, PreTone). Though many parameters were altered to create a distinct environment, we cannot rule out the possibility that an association developed between the testing room or removing the animals from their home cage and the aversive event. Further work is needed to establish whether changes in memory strength or an enhancement of generalized fear accounts for the performance of the runner and sedentary mice.
One consistent effect of wheel running that has been proposed to mediate some the pro-cognitive effects of exercise is an increase in hippocampal neurogenesis [5, 9, 25]. The present study confirmed that the runners displayed increased number of new neurons in the granular cell layer. Whether the exercise-induced increase in neurogenesis contributed to the enhanced contextual learning cannot be directly determined from the present study. Though runners showed high levels of freezing and new neuron survival at all of the time-points measured, prior work from our laboratory has shown that the exercise-induced enhancement of contextual fear learning occurs even when hippocampal neurogenesis is reduced via irradiation [5]. However, neurogenesis was reduced but not eliminated in that study therefore the reduction may not have been sufficient to block the cognitive enhancements from running. Alternatively, it is possible that the improved performance that results from wheel running may be mediated by other factors in the brain besides neurogenesis such as elevations in neurotrophic and growth factors, enhancement of long-term potentiation (LTP), and increases in angiogenesis and synaptic plasticity all of which are positively correlated with cognitive function [8, 22–24]. Additionally, exercise-induced changes in corticosterone levels may influence the cognitive benefits associated with wheel running. For instance, a recent study by Hajisoltani et al. [36] found that 10 days of voluntary wheel running increased basal plasma levels of corticosterone when measure immediately after running as well as improved spatial learning. If corticosterone levels were reduced by adrenalectomy or with a corticosterone-synthesis inhibitor the exercise-induced cognitive benefits were blocked. Given that slight increases in corticosterone are generally beneficial for cognitive function [37] the exercise-induced increase in corticosterone levels may contribute to the improvement in learning and memory. Though it remains unresolved whether the cognitive gains from running result from specific changes or are mediated by a combination of the neurobiological changes, the current findings demonstrate that increases in neurogenesis were associated with some of the running-induced enhancements in cognitive function.
Prior work indicates that hippocampal neurogenesis is required for learning a trace conditioning task. For example, Achanta et al. [30] report that irradiation-induced reduction in hippocampal neurogenesis impairs acquisition of trace fear memories. Similar deficits in trace conditioning were found when neurogenesis was inhibited with a toxin [25]. These findings indicate that basal neurogenesis is required for associating events separated in time, but whether increasing neurogenesis enhances performance in a trace conditioning task was unknown. Our results demonstrate that enhanced hippocampal neurogenesis alone is not sufficient to enhance performance on the hippocampus dependent task, trace fear conditioning. While wheel running increased the survival and the proportion of cells that differentiated into new neurons no differences in performance in the trace conditioning task were observed suggesting enhancing hippocampal neurogenesis does not translate to enhance trace fear conditioning. An alternative possibility is that the lack of an effect of wheel running on trace fear conditioning may be specific to the training procedures employed in the present study. Prior reports have shown similar levels of freezing if mice are trained with 1 or 4 trace trials in a session [21], but whether exercise mice would shown improved performance over sedentary mice with additional training is unknown. It is important to determine which tasks are improved from increased neurogenesis and which are not to understand the functional significance of adult hippocampal neurogenesis.
The current study found that both runners and sedentary mice acquired the trace procedure. However, in contrast to contextual conditioning, runners and sedentary mice showed equivalent levels of freezing during and after the tone presentation (Figure 4A). One interpretation is that wheel running has beneficial effects on selective hippocampal processes, namely acquisition of contextual information. In agreement, when runners trained on the trace procedure were placed back into the training context they showed enhanced freezing over sedentary mice (Figure 4B). Prior work has also shown that exercise does not enhance all forms of learning. For instance, wheel running was reported to have no effect on extinction of conditioned fear to a context [8]. Alternatively, wheel running may enhance delay and contextual condition by influencing the activity of the amygdala, as some report exercise-induced enhancements in both contextual and delay conditioning [7] though others fail to see effects on delay conditioning [6]. Recent work has shown that pharmacological inhibition of the amygdala impairs performance in delay and contextual conditioning, but did not disrupt trace fear conditioning when mice were trained with either 1, 2, or 4 conditioning trials [21]. Further work is needed to understand whether running influences the amygdala in fear learning. Collectively, the findings indicate that while not all cognitive processes are enhanced by wheel running but confirm that running facilitates processing of contextual information.
In summary, the present study reveals novel findings on the effects of wheel running on cognitive function. Namely, runners maintain a high level of performance in a contextual fear conditioning task regardless of the retention interval between training and testing. However, sedentary mice show an increase in freezing behavior that may result from improved memory strength or increased generalized fear that masks the advantage initially observed in the runners. Additionally, this study is the first to evaluate the effects of exercise on trace conditioning learning. Findings reveal that the benefits of exercise are not global, but rather enhance certain aspects of cognitive function that relate to processing of contextual stimuli. Elucidating the cognitive processes altered by exercise will aid in optimizing therapeutic applications and may facilitate identification of the underlying neuromechanisms.
RESEARCH HIGHLIGHTS.
Exercise enhances selective hippocampus dependent cognitive processes.
Cognitive performance in runners remains stable across time.
Increased neurogenesis may not always be associated with improved learning.
Runners and sedentary mice show similar acquisition of trace fear conditioning.
Acknowledgments
Funding: This work was supported by grants from NIH, MH083807 and DA027487 to J.S.R. Funding source had no involvement in the experimental design or interpretation of the results.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–9. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2010;108:3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMorris T, Sproule J, Turner A, Hale BJ. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: a meta-analytical comparison of effects. Physiol Behav. 2011;102:421–8. doi: 10.1016/j.physbeh.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–97. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 6.Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behav Neurosci. 2004;118:1123–7. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- 7.Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behav Brain Res. 2009;207:321–31. doi: 10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulos AM, Li V, Sterlace SS, Tokushige F, Ponnusamy R, Fanselow MS. Persistence of fear memory across time requires the basolateral amygdala complex. Proc Natl Acad Sci U S A. 2009;106:11737–41. doi: 10.1073/pnas.0905257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balogh SA, Wehner JM. Inbred mouse strain differences in the establishment of long-term fear memory. Behav Brain Res. 2003;140:97–106. doi: 10.1016/s0166-4328(02)00279-6. [DOI] [PubMed] [Google Scholar]
- 12.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–3. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–27. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Rawlins JN, Tsaltas E. The hippocampus, time and working memory. Behav Brain Res. 1983;10:233–62. doi: 10.1016/0166-4328(83)90033-5. [DOI] [PubMed] [Google Scholar]
- 15.Xavier GF, Costa VC. Dentate gyrus and spatial behaviour. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:762–73. doi: 10.1016/j.pnpbp.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 17.Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–85. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol Learn Mem. 2001;76:447–61. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- 19.Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–23. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 20.McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–46. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Raybuck JD, Lattal KM. Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLoS One. 2011;6:e15982. doi: 10.1371/journal.pone.0015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: how physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Med. 2008;10:47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- 24.Rhyu IJ, Bytheway JA, Kohler SJ, Lange H, Lee KJ, Boklewski J, McCormick K, Williams NI, Stanton GB, Greenough WT, Cameron JL. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience. 2010;167:1239–48. doi: 10.1016/j.neuroscience.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- 27.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG, Gomez-Pinedo U, Perez-Villalba A, Rosello J, Trejo JL, Barcia JA, Canales JJ. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 29.Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–54. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achanta P, Fuss M, Martinez JL., Jr Ionizing radiation impairs the formation of trace fear memories and reduces hippocampal neurogenesis. Behav Neurosci. 2009;123:1036–45. doi: 10.1037/a0016870. [DOI] [PubMed] [Google Scholar]
- 31.Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–26. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- 32.Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Haferkamp EH, Rhodes JS. Adult hippocampal neurogenesis and c-Fos induction during escalation of voluntary wheel running in C57BL/6J mice. Behav Brain Res. 2010;213:246–52. doi: 10.1016/j.bbr.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J. Alterations in fear conditioning and amygdalar activation following chronic wheel running in rats. Pharmacol Biochem Behav. 2006;84:306–12. doi: 10.1016/j.pbb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Balogh SA, Radcliffe RA, Logue SF, Wehner JM. Contextual and cued fear conditioning in C57BL/6J and DBA/2J mice: context discrimination and the effects of retention interval. Behav Neurosci. 2002;116:947–57. doi: 10.1037//0735-7044.116.6.947. [DOI] [PubMed] [Google Scholar]
- 35.Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y. Long-lasting incubation of conditioned fear in rats. Biol Psychiatry. 2009;65:881–6. doi: 10.1016/j.biopsych.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajisoltani R, Rashidy-Pour A, Vafaei AA, Ghaderdoost B, Bandegi AR, Motamedi F. The glucocorticoid system is required for the voluntary exercise-induced enhancement of learning and memory in rats. Behav Brain Res. 2011;219:75–81. doi: 10.1016/j.bbr.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y, Jadid M. Post-training administration of corticosterone enhances consolidation of contextual fear memory and hippocampal long-term potentiation in rats. Neurobiol Learn Mem. 2009;91:260–5. doi: 10.1016/j.nlm.2008.10.008. [DOI] [PubMed] [Google Scholar]