Abstract
Alcoholic and nonalcoholic steatohepatitis are characterized by fatty liver plus inflammation. It is generally believed that steatosis promotes inflammation, while inflammation in turn aggregates steatosis. Thus, we hypothesized the deletion of interleukin-10 (IL-10), a key anti-inflammatory cytokine, exacerbates liver inflammation, steatosis, and hepatocellular damage in alcoholic and nonalcoholic fatty liver disease models that were achieved via feeding mice with a liquid diet containing 5% ethanol for 4 weeks or a high fat diet for 12 weeks, respectively. IL-10 knockout (IL-10−/−) mice and several other strains of genetically modified mice were generated and used. Compared to wild-type mice, IL-10−/− mice had greater liver inflammatory response with higher levels of IL-6 and hepatic signal transducer and activator of transcription 3 (STAT3) activation, but less steatosis and hepatocellular damage after alcohol or high fat diet feeding. An additional deletion of IL-6 or hepatic STAT3 restored steatosis and hepatocellular damage but further enhanced liver inflammatory response in IL-10−/− mice. In addition, the hepatic expression of SREBP1c and key downstream lipogenic proteins and enzymes in fatty acid synthesis were downregulated in IL-10−/− mice. Conversely, IL-10−/− mice displayed enhanced levels of phosphorylated AMPK and its downstream targets including phosphorylated ACC1 and CPT-1 in the liver. Such dysregulations were corrected in IL-10−/−IL-6−/− or IL-10−/−STAT3Hep−/− double knockout mice. In conclusion, IL-10−/− mice are prone to liver inflammatory response but resistant to steatosis and hepatocellular damage induced by ethanol or high fat diet feeding. Resistance to steatosis in these mice is attributable to elevation of inflammation-associated hepatic IL-6/STAT3 activation that subsequently downregulates lipogenic genes but upregulates fatty acid oxidation-associated genes in the liver.
Keywords: STAT3, SREBP-1, ethanol, high fat diet, AMPK
Introduction
Alcoholic steatohepatitis (ASH) and nonalcoholic steatohepatitis (NASH) are the two prominent causes of chronic liver diseases worldwide, leading to liver fibrosis, cirrhosis, and hepatocellular carcinoma. Both diseases are histologically similar and characterized microscopically by steatosis, hepatocellular damage, pericellular fibrosis, and inflammation with predominantly polymorphonuclear granulocytes.1–3 At present, it is not clear why only a small percentage of patients with alcoholic and nonalcoholic fatty liver develop inflammation in the liver.4, 5 Gut-derived LPS-TLR4-Kupffer cells-TNF-α axis is generally believed to play a key role in inducing inflammation in both ASH and NASH.5–10 Both ASH and NASH patients have elevated levels of several proinflammatory cytokines in the liver and serum, including IL-8 and IL-17, which function as critical chemoattractants and activators for neutrophils and contribute to liver inflammation and injury in these diseases.11–13 Furthermore, lipid accumulation in hepatocytes induces the production of proinflammatory cytokines14–16 and hepatic lipotoxicity that promote hepatocellular damage, Kupffer cell activation, and inflammation,6, 17, 18 suggesting that steatosis promotes liver inflammation. However, the effects of inflammation on steatosis and hepatocellular damage still remain obscure. Inflammation has been implicated in promoting steatosis and liver injury via production of proinflammatory cytokines such as TNF-α and IL-1 in mice.19, 20 The lipogenic effects of TNF-α and IL-1β are mediated via upregulation of the master lipid synthesis transcription factor sterol regulatory element-binding protein 1 (SREBP-1c) 21 and the key triglyceride synthesis enzyme diacylglycerol acyltransferase,20 respectively.
In addition to producing TNF-α and IL-1β, inflammatory cells also produce hepatoprotective cytokines (such as IL-6 and IL-22) and anti-inflammatory cytokines (such as IL-10 and adiponectin) that ameliorate hepatocellular damage.22, 23 Among them, IL-10 has been shown to play the most significant role in ameliorating liver inflammation in many models.24, 25 The roles of IL-10 in ASH and NASH have been investigated but with controversial results. Hill et al. 26 reported that feeding IL-10−/− mice with alcohol in drinking water for 7 weeks enhanced LPS-induced liver inflammation and injury. Although the steatosis was not thoroughly examined in this study, the authors stated alcohol feeding induced fat accumulation in 50–75% of both wild-type and IL-10−/− mice and that LPS treatment attenuated steatosis in both groups.26 Collective results on the role of IL-10 in high fat diet (HFD)-induced steatosis and insulin resistance have been controversial. It was reported that disruption of the IL-10 gene has no effect on insulin resistance, yet another study has shown that blockage of IL-10 aggregates insulin resistance in HFD-fed mice.27, 28 Furthermore, den Boer reported that IL-10−/− mice were more susceptible to steatosis induced by feeding with a HFD diet (40% calories from fat) compared with wild-type mice.27 The results from clinical studies on the association of IL-10 polymorphisms and ASH are also inconsistent. For example, Grove et al 29 reported that heavy drinkers with the −627°A allele in the IL-10 promoter were associated with an increased risk of development of advanced ASH, whereas others did not find such association.30 Therefore, to further clarify the role of IL-10 in ASH and NASH, IL-10−/− and wild-type mice were fed a liquid diet containing 5% ethanol for 4 weeks or a HFD diet (60% calories from fat) for 12 weeks. As expected, our results show that IL-10−/− mice had greater liver inflammation, but surprisingly had less steatosis and liver injury compared with wild-type mice. We also found that in response to alcohol or HFD feeding, IL-10−/− mice produce markedly greater levels of IL-6/signal transducer and activator of transcription 3 (STAT3) activation in hepatocytes that subsequently attenuate steatosis and liver injury.
Materials and Methods
Mice, ethanol and HFD feeding models
Eight- to ten-week-old male mice were used in this study. IL-10−/−, IL-6−/−, and their wild-type control C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Generation of hepatocyte-specific STAT3 knockout (STAT3Hep−/−) mice, IL-10−/−IL-6−/−, and IL-10−/−STAT3Hep−/− double KO mice is described in the supporting document.
For chronic alcohol feeding model, mice were fed a Lieber-DeCarli diet containing 5% ethanol (ETOH) or pair-fed control diet as described in supporting document. For HFD feeding model, mice were fed HFD (60 kcal % saturated lard) obtained from Research Diets, Inc. (New Brunswick, NJ) for 12 weeks, or standard diet (STD) (10 kcal % fat) as control groups.
Statistical Analysis
Data are expressed as means ± SEM. Eight to ten mice/per group were used. To compare values obtained from two groups, the Student’s t-test was performed. To compare values obtained from three or more groups, one-way ANOVA was performed followed by Tukey’s post hoc test. A value of P < 0.05 was considered significant. Statistical analyses between STD and HFD groups in Figs. 2–6 were not performed and labeled due to too many parameters.
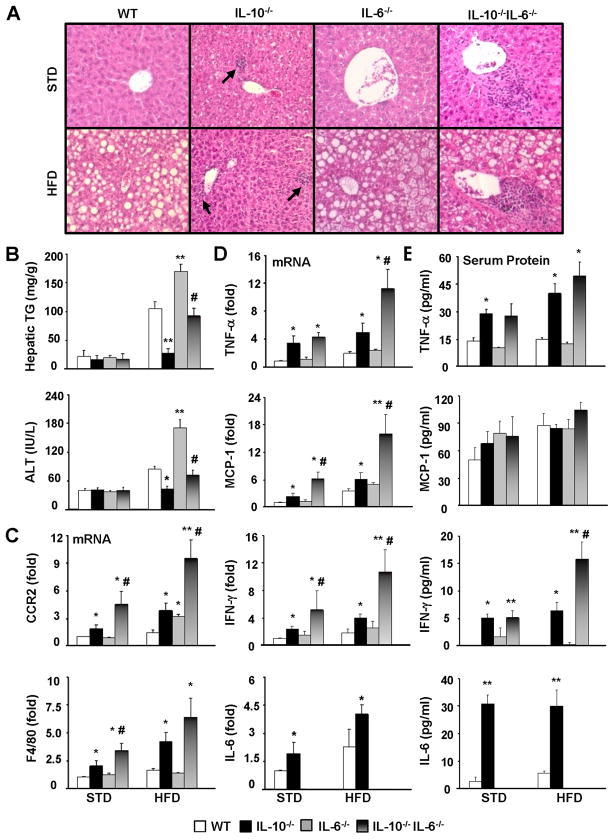
Figure 2. HFD-induced steatosis, liver inflammatory response and injury are exacerbated in IL-10−/−IL-6−/− dKO mice compared with IL-10−/− mice.
WT, IL-10−/−, IL-6−/−, IL-10−/−IL-6−/− mice were placed on a HFD or STD for 12 weeks. (A) H&E staining of liver sections. (B) Liver triglyceride and serum ALT levels. (C, D) Real-time PCR analyses of hepatic inflammatory markers CCR2 (for monocytes) and F4/80 (for macrophages) (C) and hepatic cytokines (D). (E) Serum levels of cytokines. *P<0.05, **P<0.01 in comparison with corresponding WT group; # P<0.05 in comparison with corresponding IL-10−/− group.
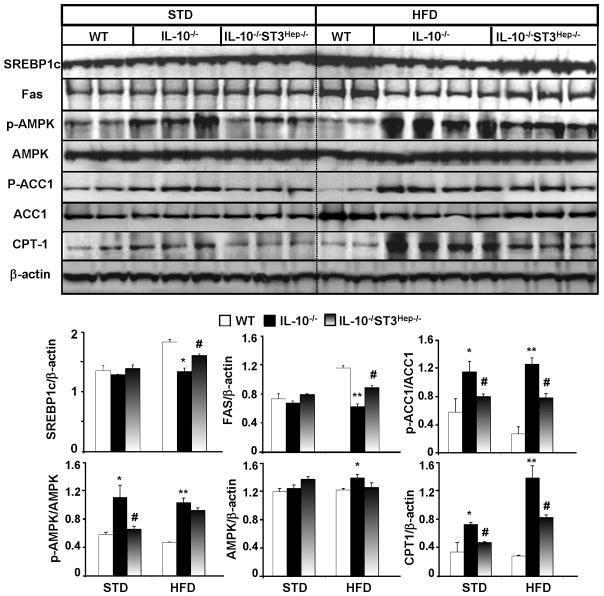
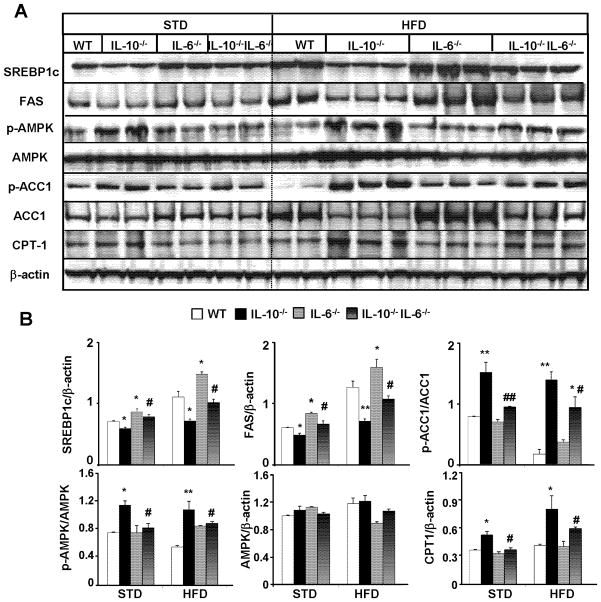
Figure 6. Downregulation and upregulation of hepatic lipogenic and fatty acid oxidation genes in HFD-fed IL-10−/− mice, respectively, which are prevented in IL-10−/−STAT3Hep−/− dKO mice.
(A) Representative Western blot analyses of lipogenic genes and fatty acid oxidative genes from the liver tissues of mice fed with STD or HFD for 12 weeks. (B) Densitometric analysis from different experiments. *P<0.05, **P<0.01 in comparison with corresponding WT group; # P<0.05, ## P<0.01 in comparison with corresponding IL-10−/− group.
Results
IL-10−/− mice are prone to ETOH and HFD-induced hepatic inflammatory response and IL-6/STAT3 activation, but resistant to steatosis development
To investigate the role of IL-10 in ASH and NASH, wild-type and IL-10−/− mice were fed ETOH diet and HFD, and their corresponding control diets. A small percentage (less than 10%) of IL-10−/− mice developed colitits with rectal prolapse during feeding and were removed from the experiments. The rest of IL-10−/− mice gained similar body weight during feeding (supplemental Figs. S1–2). In addition, IL-10−/− and wild-type mice had similar levels of serum ethanol concentrations after gavage of ethanol (supplemental Fig. S3).
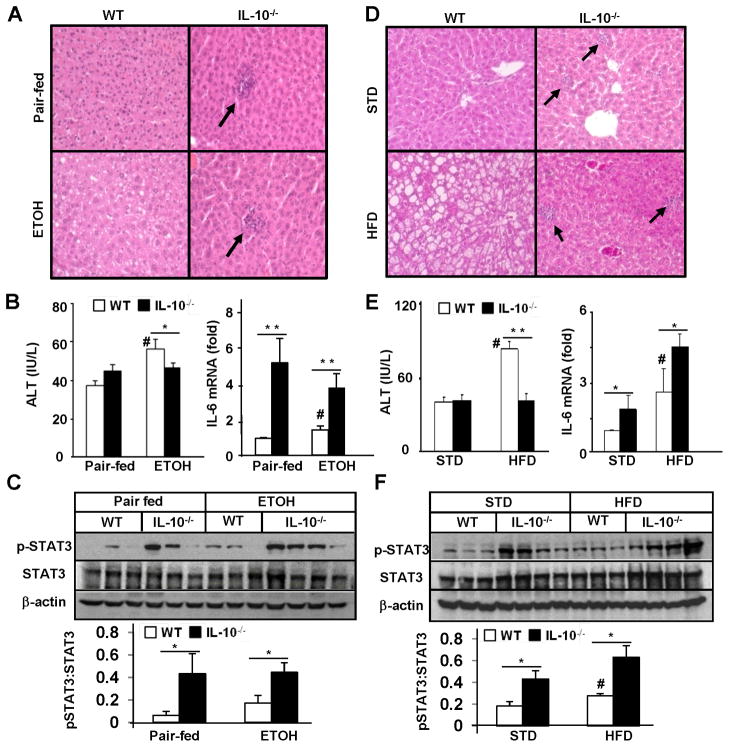
As shown in Figs. 1A–C, in wild-type mice, ETOH feeding induced significantly greater steatosis, liver injury (serum ALT levels), and elevation of hepatic IL-6 levels compared to pair-fed groups. Hepatic pSTAT3 levels also tended to be higher in ETOH-fed mice compared to pair-fed groups, but did not reach a statistically significant difference. Surprisingly, despite increased liver inflammatory responses (Figs. S4–S5), IL-10−/− mice were resistant to ETOH-induced steatosis and elevation of serum ALT (Figs. 1A–B) compared with wild-type mice. In addition, hepatic IL-6 mRNA and pSTAT3 protein levels were markedly higher in IL-10−/− mice vs. wild-type mice (Figs. 1B–C).
Figure 1. IL-10−/− mice are resistant to ETOH- and HFD-induced steatosis and liver injury but more susceptible to inflammatory response and hepatic IL-6/STAT3 activation.
Wild-type and IL-10−/− mice were fed an ETOH diet or pair-fed for 4 weeks (A-C) and in separate experiments were fed a HFD or STD for 12 weeks (D-F). (A, D) H&E staining. Note the absence of fat but increased infiltration of inflammatory cells in IL-10−/− mice. (B, E) Serum ALT and real-time PCR analyses of liver IL-6 mRNA. (C, F) Western blot analyses of liver tissues. The density of pSTAT3 and STAT3 was quantified from 3 independent experiments. *P<0.05, **P<0.01 in comparison with corresponding WT groups. # P<0.05 in comparison with corresponding pair-fed or STD-fed WT groups.
Results from HFD feeding are shown in Figs. 1D–F. In wild-type mice, HFD feeding induced markedly greater fatty liver, and elevation of ALT, hepatic IL-6 and pSTAT3 activation compared to STD feeding. IL-10−/− mice were prone to HFD-induced liver inflammatory response (supplemental Table SI, Figs. 2–3) and elevation of hepatic IL-6/pSTAT3 but resistant to HFD-induced steatosis and elevation of serum ALT compared to wild-type mice.
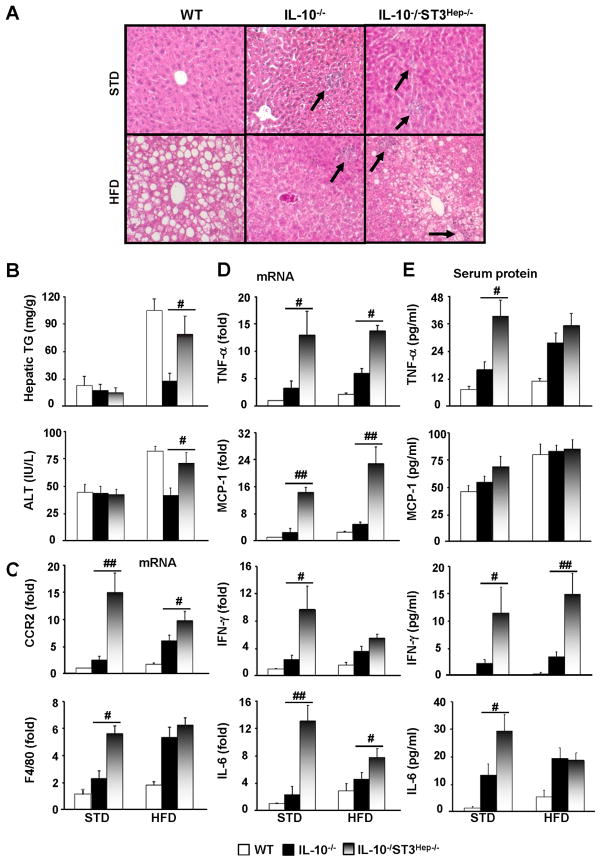
Figure 3. HFD-induced steatosis, liver inflammatory response and injury are exacerbated in IL-10−/−STAT3Hep−/− dKO mice compared with IL-10−/− mice.
WT, IL-10−/−, IL-10−/−STAT3Hep−/− mice were placed on a HFD or STD for 12 weeks. (A) H&E staining. (B) Liver triglyceride and serum ALT levels. (C, D) Real-time PCR analyses of hepatic inflammatory markers (C) and hepatic cytokines (D). (E) Serum levels of cytokines. Statistical analyses were only performed between IL-10−/−STAT3Hep−/− and IL-10−/− mice. # P<0.05, ## P<0.01.
Elevation of IL-6 contributes to the resistance of IL-10−/− mice to HFD- and ETOH-induced steatosis and hepatic injury
The hepatoprotection of IL-6/STAT3 in ameliorating fatty liver diseases has been well-documented in many rodent models.22 Therefore, we hypothesized the elevated IL-6/STAT3 activation is responsible for the resistance of IL-10−/− mice to ETOH- or HFD-induced steatosis. To test this hypothesis, we made an additional deletion of IL-6 in IL-10−/− mice to generate IL-10−/−IL-6−/− dKO mice.
Four lines of mice were fed STD or HFD for 12 weeks. As shown in Fig. 2, compared with wild-type mice, HFD-induced steatosis and serum ALT elevation were exacerbated in IL-6−/− mice but diminished in IL-10−/− mice, and the additional deletion of IL-6 restored the HFD-induced steatosis and serum ALT elevation in IL-10−/− mice to levels comparable to wild-type mice as verified by histological analysis (Fig. 2A) and by measuring hepatic triglyceride and serum ALT (Fig. 2B).
Hepatic expression of several inflammatory markers and cytokines was shown in Figs. 2C–D. In general, compared with wild-type mice, IL-6−/− mice had comparable while IL-10−/− mice had higher expression levels of these genes in both STD- and HFD-fed groups. Expression of most of these genes was exacerbated in IL-10−/−IL-6−/− dKO mice compared with IL-10−/− mice.
Fig. 2E shows the serum cytokine levels. Compared with wild-type mice, IL-6−/− mice had similar levels of serum TNF-α and IFN-γ, while IL-10−/− mice had higher levels of these cytokines in both STD and HFD groups. Serum levels of IFN-γ were further elevated in IL-10−/−IL-6−/− dKO mice vs. IL-10−/− mice after HFD feeding. Finally, serum levels of IL-6 were higher in IL-10−/− mice than those in wild-type mice. As expected, IL-6 levels were not detected in IL-6−/− and IL-10−/−IL-6−/− dKO mice.
Four lines of mice were also fed ETOH and pair-fed for 4 weeks, and analyzed similarly to the studies performed in Fig. 2. In general, similar findings to HFD model were also seen in ETOH feeding model and described in supplemental Fig. S4.
Activation of hepatocyte STAT3 contributes to the resistance of IL-10−/− mice to HFD- and ETOH-induced steatosis and hepatic injury
As illustrated in Figs. 3A–B, IL-10−/− mice were resistant to HFD-induced steatosis and serum ALT elevation compared to WT mice, which was partially restored in IL-10−/−STAT3Hep−/− dKO mice. This suggests that enhanced hepatic STAT3 activation is responsible for the reduced steatosis and liver injury in IL-10−/− mice after HFD feeding.
Furthermore, Figs. 3C–D shows that hepatic mRNA levels of several inflammatory markers and cytokines were highest in IL-10−/−STAT3Hep−/− mice, followed by IL-10−/− mice and wild-type mice in both STD and HFD-fed groups. Serum levels of TNF-α, IFN-γ, and IL-6 were also higher in IL-10−/−STAT3Hep−/− mice than those in IL-10−/− mice (Fig. 3E). Similar experiments to the HFD model described in Fig. 3 were also performed in ETOH-feeding model. Similar changes, but to a lesser extent, were observed in ETOH feeding model (supplemental Fig. S5).
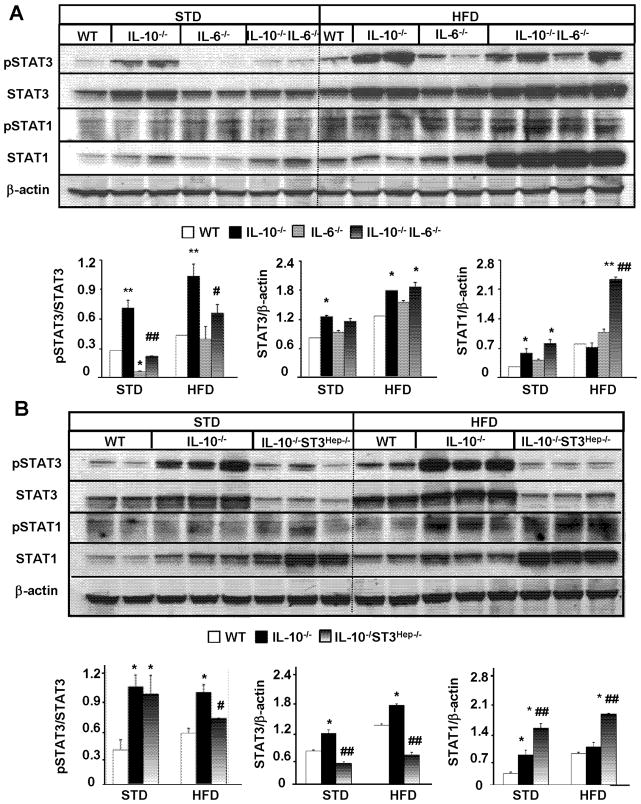
An additional deletion of IL-6 or hepatic STAT3 diminishes STAT3 activation but increases STAT1 activation in HFD-fed IL-10−/− mice
To further understand the mechanisms by which IL-10−/− mice are prone to inflammatory response but resistant to steatosis induced by HFD or ETOH diet, we examined the activation of STAT3 (pSTAT3) and pSTAT1, which play an important role in controlling steatosis and liver inflammation.31 Since the HFD model induces more dramatic phenotypes compared with the ETOH model, the studies on the underlying mechanisms were predominately focused in this HFD model.
As illustrated in Fig. 4A, in both STD- and HFD-fed groups, hepatic levels of pSTAT3 were lower in IL-6−/− mice but higher in IL-10−/− vs. wild-type mice. Compared with IL-10−/− mice, IL-10−/−IL-6−/− mice had significantly lower levels of hepatic activated pSTAT3 expression, while expression of STAT3 was comparable in these two groups. Additionally, expression of pSTAT1 and STAT1 protein was higher in the HFD-fed group vs. the STD group, with the greatest expression in IL-10−/−IL-6−/− mice.
Figure 4. An additional deletion of IL-6 or hepatic STAT3 diminishes STAT3 activation but increases STAT1 activation in HFD-fed IL-10−/− mice.
(A, B) Western blot analyses of liver tissues from different lines of mice in both STD- and HFD-fed groups. Representative Western blot pictures are shown. Densitometric analyses from different independent experiments are shown. *P<0.05, **P<0.01 in comparison with corresponding WT group; # P<0.05, ## P<0.01 in comparison with corresponding IL-10−/− group.
As expected, an additional deletion of hepatocyte STAT3 markedly reduced the expression of pSTAT3 and STAT3 in IL-10−/−STAT3Hep−/− mice compared with wild-type and IL-10−/− mice (Fig. 4B). Interestingly, expression of STAT1 protein was higher in these dKO mice compared with other groups (Fig. 4B).
IL-6 and its downstream signal STAT3 are responsible for the downregulation and upregulation of hepatic lipogenic and fatty acid oxidation genes in HFD-fed IL-10−/− mice, respectively
In agreement with previous reports, HFD feeding upregulates hepatic protein expression of nuclear mature SREBP-1c, a master transcription factor that controls lipogenic gene expression, and the expression of its target genes acetyl-CoA carboxylase (ACC1) and fatty acid synthase (FAS) (WT in HFD group vs. STD group, Fig. 5A). Hepatic expression of SREBP-1c, ACC1 and FAS was higher in IL-6−/− mice but lower in IL-10−/− mice compared with those in wild-type mice (Figs. 5A–B). Reduced expression of these genes in IL-10−/− mice was partially reversed in IL-10−/−IL-6−/− dKO mice (Figs. 5A–B).
Figure 5. Downregulation and upregulation of hepatic lipogenic and fatty acid oxidation genes in HFD-fed IL-10−/− mice, respectively, which are prevented in IL-10−/−IL-6−/− dKO mice.
(A) Representative Western blot analyses of lipogenic genes and fatty acid oxidative genes from the liver tissues of mice fed with STD or HFD for 12 weeks. (B) Densitometric analysis of the Western blots from different experiments. *P<0.05, **P<0.01 in comparison with corresponding WT group; # P<0.05, ## P<0.01 in comparison with corresponding IL-10−/− group.
Activation of adenosine monophosphate-activated protein kinase (AMPK) plays a key role in controlling lipid metabolism via phosphorylating and subsequently inhibiting ACC and suppressing the expression of ACC and FAS via down-regulation of SREBP-1c.32 ACC is an important enzyme for fatty acid synthesis, which catalyzes the first step in de novo fatty acid biosynthesis by converting acetyl CoA to malonyl CoA. Malonyl CoA acts as a potent inhibitor of fatty acid oxidation via inhibiting carnitine palmitoyltransferase 1 (CPT-1) which transports fatty acids into the mitochondria for oxidation.33, 34 As illustrated in Fig. 5, expression of activated (phosphorylated) AMPK was significantly higher in IL-10−/− mice than that in wild-type mice in both STD- and HFD-fed groups, such upregulation was diminished in IL-10−/−IL-6−/− mice. Expression of pAMPK was comparable between IL-6−/− mice and wild-type mice. Consistent with the elevated levels of pAMPK, IL-10−/− mice had higher levels of inhibited (phosphorylated) ACC1 compared with wild-type mice. Such elevated pACC1 was reduced in IL-10−/−IL-6−/− mice vs. IL-10−/− mice. In addition, hepatic expression of CPT1 was higher in HFD-fed IL-10−/− mice compared with wild-type mice. An additional deletion of IL-6 reduced hepatic CPT1 expression in IL-10−/−IL-6−/− mice vs. IL-10−/− mice.
Expression of these lipid metabolism-associated genes were also examined in wild-type, IL-10−/−, and IL-10−/−STAT3Hep−/− mice (Fig. 6). Compared to wild-type mice, IL-10−/− mice had reduced expression of SREBP-1c, ACC1, and FAS but enhanced expression of pAMPK, p-ACC1, and CPT-1 in the liver. These dysregulations were partially corrected in IL-10−/−STAT3Hep−/− mice.
Discussion
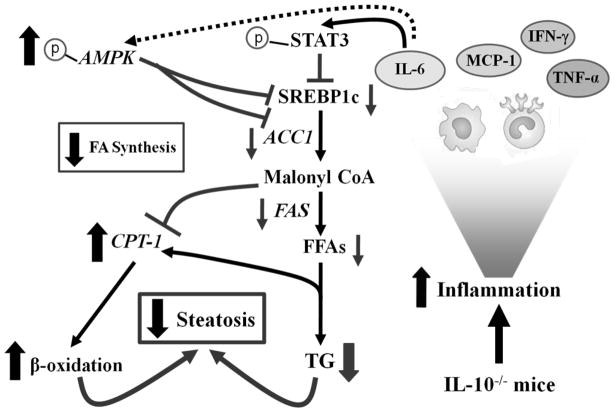
In this paper, we have demonstrated that IL-10−/− mice have greater liver inflammatory response but less steatosis and liver injury compared with wild-type mice after feeding with an ethanol or HFD diet. Our data suggest that in our models, inflammatory response reduces, rather than promotes, steatosis via activation of hepatic IL-6/STAT3 that subsequently inhibits the expression of lipogenic genes (SREBP1c, ACC1, and FAS). In concert, IL-6 upregulates the expression of CPT-1 and activates AMPK which in turn further attenuates the expression of SREBP1c and its target genes and inhibits ACC1. We have integrated our findings in a model depicting the effects of inflammation on steatosis in IL-10−/− mice (Figure 7).
Figure 7. Increased inflammation-associated IL-6/STAT3 activation ameliorates steatosis in ASH and NASH in IL-10−/− mice.
During feeding with an ETOH or a HFD diet, IL-10−/− mice are associated with increased inflammatory response and production of a variety of cytokines. Among them, TNF-α, IFN-γ, and MCP-1 may promote steatosis, liver injury and inflammation, while IL-6 activates STAT3 in hepatocytes subsequently downregulating SREBP-1c, ACC1, and FAS, resulting in the abrogation of fatty acid synthesis. IL-6 also stimulates the phosphorylation and activation of AMPK, which subsequently inhibits the expression of SREBP1c and its target genes, and phosphorylates and inhibits ACC1, collectively resulting in the stimulation of β-oxidation. It is likely that the anti-lipogenic effects of IL-6/STAT3 on hepatic steatosis dominate the lipogenic effects of other factors in IL-10−/− mice, leading to the resistance of these mice to alcohol and HFD-induced steatosis.
Several lines of evidence suggest that inflammation-associated elevation of IL-6/STAT3 contributes to reduced steatosis in ETOH or HFD-fed IL-10−/− mice. First, serum and hepatic IL-6 levels and activation of hepatic STAT3 were higher in IL-10−/− mice vs. wild-type mice (Figs. 1–4, supplemental Figs S4–5). Second, the hepatoprotection of IL-6/STAT3 in steatosis has been well-documented in both ETOH and HFD models.31, 35 Third, an additional deletion of IL-6 or hepatic STAT3 restores steatosis and liver injury in IL-10−/− mice, providing conclusive evidence that elevated IL-6/STAT3 activation contributes to the reduced steatosis and hepatocellular damage in IL-10−/− mice. Finally, it is well-established that the anti-steatotic effects of IL-6/STAT3 are mediated via inhibition of lipogenic genes (SREBP-1c, ACC, and FAS) and stimulation of fatty acid oxidation genes (pAMPK and CPT-1) in the liver. 35–37 Our results revealed that expression of these lipogenic genes and fatty acid oxidation genes were downregulated and upregulated, respectively, in IL-10−/− mice, these dysregulations were corrected after an additional deletion of IL-6 or hepatic STAT3 in dKO mice, suggesting that IL-6/STAT3 activation is responsible for inhibition of lipogenic genes and upregulation of fatty acid oxidation genes in IL-10−/− mice. The mechanism by which the IL-6/STAT3 activation mediates the decrease in lipogenic gene expression may involve the interaction of STAT3 and SREBP-1c promoter. Numerous studies have shown that activated STAT3 inhibits SREBP-1c promoter activity in hepatocytes 38 and results in decreased SREBP-1c protein expression,35–37 suggesting that STAT3 activation can directly inhibit SREBP-1c promoter activity and subsequently attenuate SREBP-1c-controlled lipogenic genes. However, how STAT3 inhibits SREBP-1c promoter activity remains unknown.
While IL-10 is a well-documented anti-inflammatory cytokine,39 IL-6 acts as a pro-inflammatory cytokine in various conditions.40 In the liver, IL-6 is implicated in promoting liver inflammation via activation of hepatic STAT3 and subsequent production of acute phase proteins in various liver injury models.41 Interestingly, an additional deletion of IL-6 or hepatic STAT3 exacerbated rather than reduced liver inflammatory response in IL-10−/− mice (Figs. 1–3), suggesting that IL-6 acts as an anti-inflammatory cytokine via activation of hepatocyte STAT3 in IL-10−/− mice in our models. By using hepatocyte-specific IL-6 receptor knockout mice, Wunderlich et al recently also reported that IL-6 acts as an anti-inflammatory cytokine via targeting hepatocytes.42 One potential explanation for the anti-inflammatory effect of IL-6/STAT3 in our models is due to its hepatoprotection in reducing steatosis and liver injury, and subsequently preventing steatosis/injury-associated inflammation. Another explanation for the anti-inflammatory effect of IL-6/STAT3 is mediated via inhibition of hepatic STAT1, a key signaling pathway to promote liver inflammation.43 As shown in Fig. 4, hepatic expression of activated pSTAT1 was markedly higher in HFD-fed IL-10−/−IL-6−/− and IL-10−/−STAT3Hep−/− dKO mice compared with IL-10−/− mice, indicating IL-6/STAT3 activation is responsible for inhibiting STAT1 activation.
In summary, IL-10−/− mice displayed greater liver inflammatory response but less steatosis after ETOH or HFD feeding compared with wild-type mice, and inflammation-associated IL-6/STAT3 activation contributes to the reduced steatosis in these mice. Interestingly, hepatic IL-6/STAT3 is also activated in wild-type mice after ETOH or HFD feeding, but to a lesser extent compared with IL-10−/− mice (Figs. 1–3). This suggests that endogenous IL-10 plays an important role in inhibiting hepatic IL-6/STAT3 activation, which may account for the weak activation of this signaling pathway in the liver in wild-type mice during ETOH or HFD feeding. It was reported that hepatic levels of IL-10 were elevated in the mice after 8-week HFD feeding;28 however, we did not observe hepatic IL-10 upregulation in wild-type mice after 12-week HFD or 4-week ETOH feeding. In contrast, we observed marked upregulation of hepatic IL-10 mRNA in mice fed with HFD for one year (unpublished data). Furthermore, it has been reported that hepatic expression of IL-10 mRNA is not upregulated in obese individuals without fatty liver but markedly upregulated in those with fatty liver, which is further increased in individuals with NASH.44 This suggests that hepatic IL-10 is elevated after long-term HFD consumption in patients and in mice, which may play a compensatory role in preventing inflammation in fatty liver disease.
The fact that IL-10−/− mice had greater liver inflammatory response but less steatosis suggests that inflammation, as reported previously, may not only promote the development of fatty liver via producing TNF-α and IL-1 but may also ameliorate the fatty liver via producing cytokines (such as IL-6) that activate STAT3. Therefore, the overall effect of inflammation on hepatic steatosis is determined by the balance between detrimental cytokines that promote steatosis and hepatoprotective cytokines that prevent steatosis. It is of keen interest to explore the effect of inflammation on steatosis in patients with ASH and NASH. Recently, Bertola et al 44 reported that the liver of obese patients without obvious steatosis (S0) was associated with elevation IL-6 but not TNF-α and IL-1β. It is plausible that such elevation of inflammation-associated IL-6 plays a compensatory role in preventing the development of steatosis in the early stage of nonalcoholic fatty liver in obese patients. The liver of obese patients with severe steatosis (S3) and NASH was associated with highest fold induction of IL-6, followed by TNF-α and IL-1β. It is probable that the steatosis in these patients is modulated negatively by IL-6 but positively by TNF-α and IL-1β. Modulation of the balance between these cytokines may be a therapeutic option to treat NAFLD and NASH.
Supplementary Material
Acknowledgments
This work was supported by the intramural program of NIAAA, NIH.
List of Abbreviations
- ACC1
acetyl-CoA carboxylase
- ALT
alanine aminotransferase
- AMPK
adenosine monophosphate-activated protein kinase
- ASH
alcoholic steatohepatitis
- CCR2
chemokine (C-C motif) receptor 2
- CPT-1
carnitine palmitoyltransferase I
- FAS
fatty acid synthase
- IFN-γ
interferon-γ
- HFD
high fat diet
- IL-10−/− mice
IL-10 knockout mice
- IL-10−/−IL-6−/− mice
IL-10 and IL-6 double knockout mice
- IL-10−/−STAT3Hep−/− or IL-10−/−ST3Hep−/− mice
IL-10 and hepatocyte-specific STAT3 double knockout mice
- MCP-1
Monocyte chemotactic protein-1
- NASH
nonalcoholic steatohepatitis
- STAT3
signal transducer and activator of transcription 3
- STAT3Hep−/− mice
hepatocyte-specific STAT3 knockout mice
- STD
standard diet
- SREBP1c
sterol regulatory element-binding protein 1
- TGF-β1
transforming growth factor beta1
- TNF-α
tumor necrosis factor-alpha
Footnotes
No conflicts of interest exist for all authors.
References
- 1.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 2.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–69. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 3.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–98. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 4.Day CP. Who gets alcoholic liver disease: nature or nurture? J R Coll Physicians Lond. 2000;34:557–62. [PMC free article] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–46. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 6.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–88. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 7.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–44. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–9. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–66. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurman RG, Bradford BU, Iimuro Y, Knecht KT, Arteel GE, Yin M, Connor HD, Wall C, Raleigh JA, Frankenberg MV, Adachi Y, Forman DT, Brenner D, Kadiiska M, Mason RP. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13 (Suppl):S39–50. doi: 10.1111/jgh.1998.13.s1.39. [DOI] [PubMed] [Google Scholar]
- 11.Lemmers A, Moreno C, Gustot T, Marechal R, Degre D, Demetter P, de Nadai P, Geerts A, Quertinmont E, Vercruysse V, Le Moine O, Deviere J. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–57. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 12.Hill DB, Marsano LS, McClain CJ. Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology. 1993;18:576–80. [PubMed] [Google Scholar]
- 13.Dominguez M, Miquel R, Colmenero J, Moreno M, Garcia-Pagan JC, Bosch J, Arroyo V, Gines P, Caballeria J, Bataller R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–50. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 14.Joshi-Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, Cave M, Hote P, McClain CJ. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46:823–30. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- 15.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–94. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 16.Bigorgne AE, Bouchet-Delbos L, Naveau S, Dagher I, Prevot S, Durand-Gasselin I, Couderc J, Valet P, Emilie D, Perlemuter G. Obesity-induced lymphocyte hyperresponsiveness to chemokines: a new mechanism of Fatty liver inflammation in obese mice. Gastroenterology. 2008;134:1459–69. doi: 10.1053/j.gastro.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 17.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445–51. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–9. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–52. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 20.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–34 e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawler JF, Jr, Yin M, Diehl AM, Roberts E, Chatterjee S. Tumor necrosis factor-alpha stimulates the maturation of sterol regulatory element binding protein-1 in human hepatocytes through the action of neutral sphingomyelinase. J Biol Chem. 1998;273:5053–9. doi: 10.1074/jbc.273.9.5053. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011;7:536–50. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–69. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 24.Douglas DB, Beiting DP, Loftus JP, Appleton JA, Bliss SK. Combinatorial effects of interleukin 10 and interleukin 4 determine the progression of hepatic inflammation following murine enteric parasitic infection. Hepatology. 2010;51:2162–71. doi: 10.1002/hep.23576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis H, Le Moine O, Peny MO, Gulbis B, Nisol F, Goldman M, Deviere J. Hepatoprotective role of interleukin 10 in galactosamine/lipopolysaccharide mouse liver injury. Gastroenterology. 1997;112:935–42. doi: 10.1053/gast.1997.v112.pm9041256. [DOI] [PubMed] [Google Scholar]
- 26.Hill DB, D’Souza NB, Lee EY, Burikhanov R, Deaciuc IV, de Villiers WJ. A role for interleukin-10 in alcohol-induced liver sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res. 2002;26:74–82. [PubMed] [Google Scholar]
- 27.den Boer MA, Voshol PJ, Schroder-van der Elst JP, Korsheninnikova E, Ouwens DM, Kuipers F, Havekes LM, Romijn JA. Endogenous interleukin-10 protects against hepatic steatosis but does not improve insulin sensitivity during high-fat feeding in mice. Endocrinology. 2006;147:4553–8. doi: 10.1210/en.2006-0417. [DOI] [PubMed] [Google Scholar]
- 28.Cintra DE, Pauli JR, Araujo EP, Moraes JC, de Souza CT, Milanski M, Morari J, Gambero A, Saad MJ, Velloso LA. Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. J Hepatol. 2008;48:628–37. doi: 10.1016/j.jhep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Grove J, Daly AK, Bassendine MF, Gilvarry E, Day CP. Interleukin 10 promoter region polymorphisms and susceptibility to advanced alcoholic liver disease. Gut. 2000;46:540–5. doi: 10.1136/gut.46.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardet JP, Scherman E, Costa C, Campillo B, Bories PN. Combined polymorphisms of tumour necrosis factor alpha and interleukin-10 genes in patients with alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2006;18:673–9. doi: 10.1097/00042737-200606000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Gao B. Cytokines, STATs and liver disease. Cell Mol Immunol. 2005;2:92–100. [PubMed] [Google Scholar]
- 32.Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Enjoji M, Takayanagi R, Nakamuta M. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21:507–11. [PubMed] [Google Scholar]
- 33.Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol. 2006;574:41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saggerson D. Malonyl-CoA, a key signaling molecule in mammalian cells. Annu Rev Nutr. 2008;28:253–72. doi: 10.1146/annurev.nutr.28.061807.155434. [DOI] [PubMed] [Google Scholar]
- 35.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, Osei-Hyiaman D, Moh A, Fu XY, Pacher P, Kunos G, Gao B. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–58. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroy DC, Beraza N, Tschaharganeh DF, Sander LE, Erschfeld S, Giebeler A, Liedtke C, Wasmuth HE, Trautwein C, Streetz KL. Lack of interleukin-6/glycoprotein 130/signal transducers and activators of transcription-3 signaling in hepatocytes predisposes to liver steatosis and injury in mice. Hepatology. 2010;51:463–73. doi: 10.1002/hep.23322. [DOI] [PubMed] [Google Scholar]
- 37.Inoue H, Ogawa W, Ozaki M, Haga S, Matsumoto M, Furukawa K, Hashimoto N, Kido Y, Mori T, Sakaue H, Teshigawara K, Jin S, Iguchi H, Hiramatsu R, LeRoith D, Takeda K, Akira S, Kasuga M. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med. 2004;10:168–74. doi: 10.1038/nm980. [DOI] [PubMed] [Google Scholar]
- 38.Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci U S A. 2004;101:10422–7. doi: 10.1073/pnas.0402511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–68. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 41.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–68. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wunderlich FT, Strohle P, Konner AC, Gruber S, Tovar S, Bronneke HS, Juntti-Berggren L, Li LS, van Rooijen N, Libert C, Berggren PO, Bruning JC. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 2010;12:237–49. doi: 10.1016/j.cmet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, Nguyen VA, Gao B. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–13. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertola A, Bonnafous S, Anty R, Patouraux S, Saint-Paul MC, Iannelli A, Gugenheim J, Barr J, Mato JM, Le Marchand-Brustel Y, Tran A, Gual P. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One. 2010;5:e13577. doi: 10.1371/journal.pone.0013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.