Abstract
OBJECTIVE
The role of uncoupling protein 2 (UCP2) in pancreatic β-cells is highly debated, partly because of the broad tissue distribution of UCP2 and thus limitations of whole-body UCP2 knockout mouse models. To investigate the function of UCP2 in the β-cell, β-cell–specific UCP2 knockout mice (UCP2BKO) were generated and characterized.
RESEARCH DESIGN AND METHODS
UCP2BKO mice were generated by crossing loxUCP2 mice with mice expressing rat insulin promoter-driven Cre recombinase. Several in vitro and in vivo parameters were measured, including respiration rate, mitochondrial membrane potential, islet ATP content, reactive oxygen species (ROS) levels, glucose-stimulated insulin secretion (GSIS), glucagon secretion, glucose and insulin tolerance, and plasma hormone levels.
RESULTS
UCP2BKO β-cells displayed mildly increased glucose-induced mitochondrial membrane hyperpolarization but unchanged rates of uncoupled respiration and islet ATP content. UCP2BKO islets had elevated intracellular ROS levels that associated with enhanced GSIS. Surprisingly, UCP2BKO mice were glucose-intolerant, showing greater α-cell area, higher islet glucagon content, and aberrant ROS-dependent glucagon secretion under high glucose conditions.
CONCLUSIONS
Using a novel β-cell–specific UCP2KO mouse model, we have shed light on UCP2 function in primary β-cells. UCP2 does not behave as a classical metabolic uncoupler in the β-cell, but has a more prominent role in the regulation of intracellular ROS levels that contribute to GSIS amplification. In addition, β-cell UCP2 contributes to the regulation of intraislet ROS signals that mediate changes in α-cell morphology and glucagon secretion.
Uncoupling protein 2 (UCP2) was discovered based on sequence homology to UCP1 (1), a well-studied UCP involved in thermogenesis. UCP1 induces a strong proton leak in the inner mitochondrial membrane, which dramatically dissipates the proton motive force (PMF), consequently halting the driving force for ATP production and dissipating energy as heat (2). Despite homology to UCP1, the precise physiological function of UCP2 remains unclear (3). A mild metabolic uncoupling function whereby UCP2 facilitates a proton leak, particularly when activated by superoxide or lipid peroxidation products, has been demonstrated (4–6); however, evidence exists that disputes this classical metabolic uncoupling function (7–9).
A growing body of evidence now suggests that UCP2 contributes to the control of mitochondrial-derived reactive oxygen species (ROS) production (3,4,10,11). This may provide an important mechanism to fine-tune mitochondria-generated ROS signals that regulate cell function and/or to prevent oxidative stress, a condition that results from chronic ROS accumulation and ultimately leads to oxidative damage and cytotoxicity (12,13).
To combat oxidative stress, β-cells express relatively high amounts of the superoxide dismutase (SOD) family of antioxidants (∼50% of that found in liver), which convert superoxide into hydrogen peroxide (H2O2), yet β-cells have relatively low expression of H2O2-scavenging enzymes (1% of that found in liver) (14). Some argue that this makes β-cells particularly susceptible to oxidative stress and cytotoxicity, whereas others argue that this creates an environment highly sensitive to ROS-related signaling. Since ROS production is directly coupled to the metabolic rate in most tissues (15), ROS could provide a vital regulatory link between glucose metabolism and insulin secretion (16–18), and UCP2 may be an important regulator of such ROS-related signals.
Since its discovery, numerous studies have demonstrated a negative link between UCP2 and β-cell function (1). UCP2 expression is upregulated in response to chronic high glucose (19,20) and fatty acid exposure (19,21–23) and is thus associated with obesity, hyperglycemia, and type 2 diabetes. More recently, mutations in the gene expressing UCP2 have been directly associated with congenital hyperinsulinemia in humans, further demonstrating this link between UCP2 and insulin secretion (24). Approximately a decade ago, whole-body UCP2 knockout (UCP2KO) mice were created on a mixed 129/SVJxC57BL/6 background (25) to explore UCP2 function in the β-cell. UCP2KO mice have reduced blood glucose levels, improved glucose tolerance, higher islet ATP content, enhanced glucose-stimulated insulin secretion (GSIS) (25), and increased intracellular ROS levels in islet cells (26,27) compared to control mice. Similar results have been demonstrated in rat insulinoma β-like cells (INS-1E), where acute knockdown of UCP2 also increased intracellular ROS and enhanced GSIS (18). However, this view of UCP2 as a negative regulator of GSIS has not been consistently supported. Backcrossing UCP2KO mice for several generations onto highly congenic background strains resulted in increased oxidative stress and impaired GSIS (28). Although the precise contribution of genetic background to these disparate effects of UCP2 on GSIS is currently unknown and is an issue that requires cautious interpretation of results, it seems that UCP2 commonly regulates ROS in all strains, further highlighting the importance of UCP2 in ROS regulation.
Until now, whole-body UCP2KO mouse models have been widely used to study the role of UCP2 in β-cell function (23,25–28). However, these models can be problematic because UCP2 deletion in other tissues and cell types, including brain and other islet cells (i.e., α-cells) (25,29,30), can affect glucose-sensing and glucose homeostasis (31,32). To elucidate the function of UCP2 in the β-cell, we have created and characterized a novel β-cell–specific UCP2KO mouse (UCP2BKO). Here, we show that UCP2 does not behave as a true uncoupler in the β-cell, but rather contributes to the regulation of β-cell ROS, which in turn regulates GSIS. In addition, we suggest that β-cell UCP2 regulates intraislet ROS signals that can target and regulate the function of neighboring glucagon-secreting α-cells.
RESEARCH DESIGN AND METHODS
Animals.
The loxUCP2 mice were a gift from Dr. Bradford Lowell (32). β-Cell–specific UCP2 deletion was accomplished by crossing loxUCP2 mice with rat insulin promoter–driven Cre recombinase (RIPCre) mice (The Jackson Laboratory, Bar Harbor, ME). Mice were genotyped using standard PCR of ear notch DNA (Fig. 1B). RIPCre mice were chosen as controls for experimentation because RIPCre and floxed mice (mice that express the floxed Ucp2 gene without Cre) gave similar results (Supplementary Fig. 1). All mice (10–13 weeks old) were age- and sex-matched and maintained on a 129J-C57BL/6-mixed background. Unless otherwise indicated, male mice were used. All animal experiments were approved by the University of Toronto Animal Care Committee, and animals were handled according to the guidelines of the Canadian Council of Animal Care. Human islets from healthy donors were isolated using the Edmonton protocol and provided by the ABCC Human Islet Distribution Program (University of Alberta, Edmonton, AB, Canada). Donation was approved by the local institutional review board.
FIG. 1.

Effective deletion of Ucp2 specifically from pancreatic β-cells using a Cre-lox recombination strategy. A: Targeting construct for the Ucp2 gene shows the floxed region (flanked by loxP sites) and the Ucp2 allele after Cre recombinase excision. Homozygote loxUCP2 mice that express Cre undergo recombination of the DNA between loxP sites, deleting exons 3 and 4, which contains the start codon. B: Typical PCR results from mouse genotyping for loxUCP2 (floxing) and Cre expression. C: Quantitative PCR results show reduced Ucp2 mRNA expression in isolated UCP2BKO islets compared with RIPCre islets. Ucp2 mRNA expression was calculated as a percentage of β-actin. The error bar shows the SEM. D: Standard PCR of various tissues shows full-length UCP2 transcripts and truncated UCP2 (Δ) where exons 3 and 4 have been removed by Cre recombinase activity. Wt, wild type. E, Two left panels: Dispersed pancreatic islets isolated from RIPCre and UCP2BKO mice immunostained for UCP2 (red) and insulin (green). Two right panels: UCP2BKO dispersed islet cells stained for Cre (red) and insulin (green) show nuclear localization of Cre recombinase. The yellow coloring represents colocalization of red and green fluorescence. n = 5–7. *P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)
Antibodies and reagents.
RedTaq polymerase for Multiplex PCR, N-acetyl-l-cysteine (NAC), and diethyl maleate (DEM) were obtained from Sigma-Aldrich (St. Louis, MO). The Cre antibody was from Covance (Emeryville, CA), and the UCP2 antibody was from Everest Biotech (Oxfordshire, U.K.). Guinea pig anti-swine insulin (Dako, Glostrup, Denmark), fluorescein isothiocyanate (FITC), and cyanine (Cy5)-labeled secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used. Rhodamine 123, and 2′,7′-dichlorodihydro fluorescein diacetate (CM-H2-DCFDA) were from Molecular Probes (Invitrogen Canada Inc., Burlington, ON, Canada).
Islet dispersion, immunohistochemistry, and confocal microscopy.
Islets were isolated from pancreata, dispersed into individual islet cells, and plated onto glass coverslips, as previously described (29). Dispersed islets were costained for insulin and UCP2 or insulin and Cre, as previously described (29).
Quantitative real-time PCR.
Quantitative PCR was performed, as previously described (33,34). Primers are listed in Supplementary Table 1. Data were normalized to β-actin mRNA.
Mitochondrial membrane potential and islet ATP content.
Mitochondrial membrane potential (ΔΨm) was quantified in dispersed β-cells, as previously reported (29). For ATP content, 8–20 islets per condition were washed with ice-cold Kreb’s Ringer buffer (KRB) and preincubated at 37°C in 2.8 mmol/L glucose for 1 h. Tubes were put in ice water, and the 2.8 mmol/L glucose buffer was replaced with ice-cold 2.8 or 16.7 mmol/L glucose KRB and incubated for 30 min at 37°C. Islets were lysed with 0.1 mol/L NaOH/0.5 mmol/L EDTA and stored at −80°C until assayed with a luciferase ATP assay kit according to the manufacturer’s instructions (Sigma-Aldrich).
Islet oxygen consumption measurements.
Isolated islets were resuspended in warm assay media (3 mmol/L glucose, 0.8 mmol/L Mg2+, 1.8 mmol/L Ca2+, 143 mmol/L NaCl, 5.4 mmol/L KCl, 0.91 mmol/L NaH2PO4, 15 mg/mL phenol red, and 1% FBS [Seahorse Bioscience, Billerica, MA]). Fifty to 80 islets were plated by pipetting 2 × 50 µL stirred islet mix into each of the 20 wells in an islet plate loaded previously with 400 µL of media and incubated 60 min at 37°C before loading into an XF24 respirometry machine (Seahorse Bioscience). Oxygen consumption rates (OCRs) were measured at 3 and 20 mmol/L glucose as well as in the presence of 5 µmol/L oligomycin (Sigma-Aldrich). Bright-field images were taken of each well with an Olympus SZX16 stereomicroscope (Olympus, Tokyo, Japan). Islet diameter was measured with Metamorph image analysis software (Molecular Devices, Sunnyvale, CA).
Oral glucose tolerance test.
After a 16-h fast, mice were gavaged with glucose (2 g/kg body wt). Blood glucose was measured at 0, 10, 20, 30, 60, and 120 min. Plasma insulin was measured at 0, 10, 20, and 60 min using an enzyme-linked immunosorbent assay kit (ALPCO Diagnostics, Salem, NH). Plasma glucagon was measured at 0 and 10 min by radioimmunoassay (RIA) kits (Millipore, Etobicoke, ON, Canada).
Insulin tolerance test.
After a 4-h fast, insulin (0.75 IU/kg body wt) was injected intraperitoneally. Blood glucose was measured at 0, 15, 30, 60, and 120 min.
Pancreatic morphology.
Pancreata were removed from anesthetized mice and fixed with 10% buffered formalin for 48 h, sectioned, and stained, as described previously (35). The ImageScope program (Aperio Technologies, Vista, CA) was used to analyze insulin- and glucagon-positive areas as well as islet density and surface area. All data were normalized to total pancreatic slice area.
GSIS by perifusion.
Islets were cultured overnight in 11 mmol/L glucose RPMI 1640 media before preincubation in 2.8 mmol/L glucose KRB buffer for 1 h. Dynamic insulin secretion was assessed by islet perifusion, as previously described (34).
Static hormone secretion and total hormone content.
Eight to 20 islets (mouse or human) per condition were washed with ice-cold KRB and preincubated at 37°C in 2.8 mmol/L glucose (±5 mmol/L DEM or 0.2 mmol/L NAC or 50 µmol/L genipin) for 1 h. Tubes were put in ice water, and the 2.8 mmol/L glucose buffer was replaced with ice-cold 2.8 or 16.7 mmol/L glucose KRB (±5 mmol/L DEM or 0.2 mmol/L NAC or 50 µmol/L genipin) and incubated for 30 min at 37°C. Insulin and glucagon secretion were measured using RIA kits (Millipore) and normalized to islet number. Total insulin and glucagon content in islets was measured by RIA (Millipore) and normalized to DNA content.
Intracellular ROS measurements.
Intracellular H2O2 was measured in dispersed β-cells and islets cultured in RPMI 1640 medium with 11 mmol/L glucose using CM-H2-DCFDA, as previously reported (27).
Statistics.
Statistical significance was assessed by two-tailed Student t test or one- or two-way ANOVA for repeated measures, followed by a Bonferroni post-test comparisons using Prism 4 software (GraphPad, San Diego, CA). A value of P < 0.05 was considered significant. All data are expressed as the mean ± SEM.
RESULTS
Knockdown of UCP2 transcript and protein in pancreatic β-cells.
UCP2 expression was reduced by 75% in UCP2BKO compared with RIPCre islets using the Cre-lox recombination system (Fig. 1C). UCP2 expression in the α-cell and other islet cell types contributes to detectable UCP2 in whole islets (29). Therefore, islets were dispersed and subjected to immunohistochemistry to confirm deletion in β-cells. UCP2 protein (red) was detectable in <10% of insulin-positive cells (green) in UCP2BKO dispersed islets, suggesting ∼90% deletion in β-cells (Fig. 1E, two left panels). Insulin-positive cells (green) were also immunostained for Cre protein (red), which was found localized to the nuclei of UCP2BKO β-cells only (Fig. 1E, two right panels) and not in floxed control β-cells (data not shown). In addition, UCP2 deletion was examined in various tissues using standard PCR. No deletion in spleen was observed; however, low but detectable deletion was seen in hypothalami from UCP2BKO mice (Fig. 1D). No UCP2 deletion was observed in any RIPCre tissue (Fig. 1D).
UCP2 deficiency in β-cells mildly increases glucose-induced ΔΨm without impact on mitochondrial coupling or islet ATP content.
To determine the contribution of UCP2 to mitochondrial uncoupling, the total OCR and OCR in the presence of saturating amounts of oligomycin were measured, the latter representing the uncoupled fraction of respiration. UCP2BKO and RIPCre islets exhibited similar uncoupled OCRs (Fig. 2C) under both basal and glucose-stimulated conditions, suggesting that UCP2 deficiency in the β-cell does not increase mitochondrial coupling. Interestingly, UCP2BKO islets showed higher total OCR compared with RIPCre islets (Fig. 2D), with both genotypes experiencing approximately 1.5-fold increased OCR upon stimulation with 20 mmol/L glucose (Fig. 2E). No difference in islet size was observed between the two groups (Supplementary Fig. 5).
FIG. 2.
Glucose-induced mitochondrial membrane hyperpolarization is mildly increased in UCP2BKO β-cells, while islet ATP content and uncoupled respiration rates remain unchanged. A: Representative traces of ΔΨm measurements using rhodamine123 in selected large cells (β-cells) from dispersed islets isolated from UCP2BKO and RIPCre mice. ΔΨm was measured initially in the presence of a low (2.8 mmol/L) glucose concentration. Membrane hyperpolarization was induced by the addition of high (20 mmol/L) glucose concentration, and 5 mmol/L sodium azide (NaN3) was used to completely depolarize the mitochondrial membrane to ensure membrane integrity. n = 5 mice/genotype. RFU, relative fluorescence units. B: Measurements of ΔΨm were normalized to basal fluorescence in the presence of low (2.8 mmol/L) glucose concentration and then expressed relative to RIPCre control ΔΨm levels. Δ1, the change in mitochondrial membrane hyperpolarization in response to increased glucose concentration. Δ2, the change in mitochondrial membrane depolarization in response to NaN3 above basal mitochondrial membrane potential. (20–30 cells/coverslip with 3 coverslips per animal were measured.) n = 5 mice/genotype. ***P < 0.001. C: Uncoupled OCR is unchanged in UCP2BKO islets. OCR was measured under saturating concentrations of oligomycin (5 µmol/L) with 3 or 20 mmol/L glucose. Measurements shown are after steady state was achieved. n = 3 separate experiments, with 13–15 measurements per condition. D: Basal OCR is increased in UCP2BKO islets. Measurements are steady state in 3 mmol/L glucose. **P < 0.01. n = 3 separate experiments with 27 (control) and 30 (UCP2BKO) measurements per condition. E: Glucose-stimulated oxygen consumption (expressed as a percentage of the basal OCR) is similar between UCP2BKO and RIPCre islets. Both genotypes experience a 1.5-fold increase in OCR after stimulation with glucose. OCRs were measured in 3 and 20 mmol/L glucose. Measurements were taken after steady state was achieved. n = 4 separate experiments with 18 (control) and 20 (UCP2BKO) measurements per condition. F: Islet ATP content in RIPCre and UCP2BKO islets cultured overnight and incubated in low (2.8 mmol/L) or high (16.7 mmol/L) glucose for 30 min. n = 3 mice/genotype; *P < 0.05. LG, low glucose; HG, high glucose. The error bars show the SEM.
UCP2BKO and RIPCre islets have similar amounts of ATP, both in low and high glucose conditions (Fig. 2F), which is reflective of these two genotypes having similar rates of uncoupled respiration. Basally (2.8 mmol/L glucose), RIPCre and UCP2BKO β-cells also have similar ΔΨm, further supporting the notion that UCP2 does not contribute to classical proton leak in β-cells (Fig. 2A and B); however, in the presence of 20 mmol/L glucose, UCP2BKO β-cells undergo very mild, yet significantly increased mitochondrial membrane hyperpolarization compared with RIPCre β-cells Fig. 2A and B), which is potentially a consequence of the greater respiratory flux observed in these cells.
UCP2 deficiency in β-cells raises intracellular ROS levels.
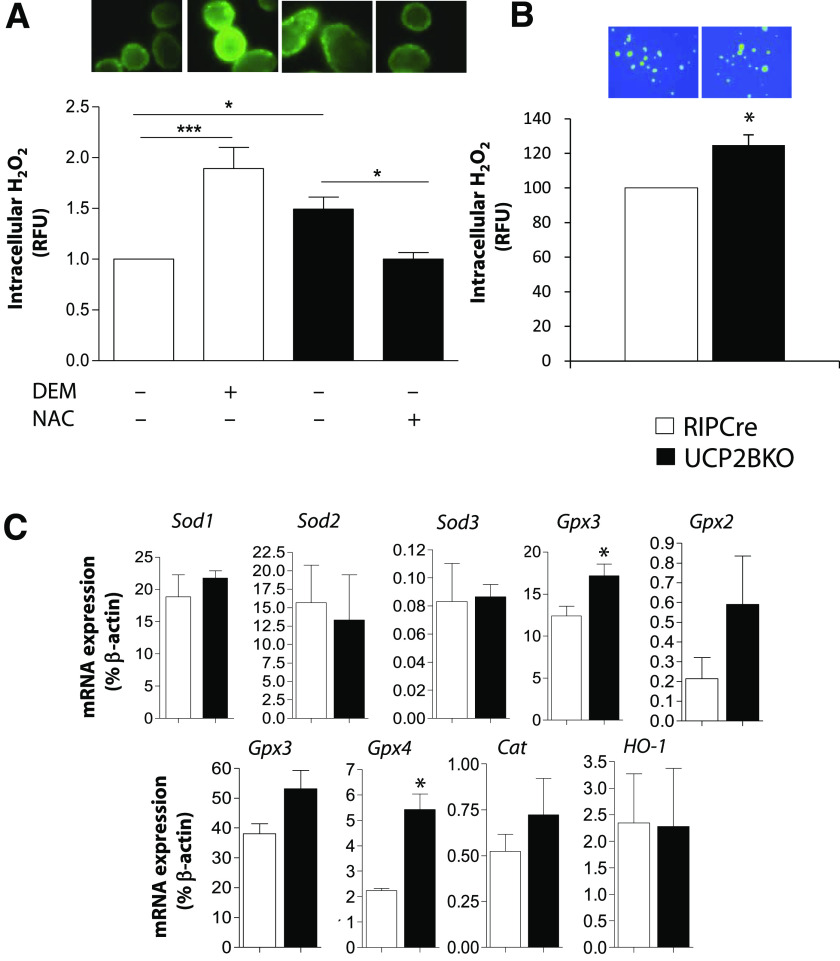
To further investigate the function of UCP2 in β-cells, a cell-permeable fluorescent H2O2 indicator (CM-H2-DCFDA), was used to measure intracellular ROS levels. Similar to that shown in islets from whole-body KO mice (26–28), UCP2BKO isolated islets (Fig. 3A) and β-cells (Fig. 3B) displayed significantly higher intracellular H2O2 levels compared with RIPCre control mice, demonstrating that UCP2 plays a role in the regulation of intracellular ROS levels in the β-cell. Subsequently, basal intracellular ROS levels were correlated with the expression of several ROS-responsive genes by quantitative PCR (Fig. 3C). In UCP2BKO islets, upregulated expression of the H2O2-scavenging glutathione peroxidase 1 (Gpx1) and Gpx4 genes was observed (Fig. 3C). In addition, Gpx2, Gpx3, and catalase (Cat) all showed a trend toward higher gene expression in UCP2BKO islets, but these values did not reach statistical significance. Interestingly, the expression of heme oxygenase-1 (HO-1), which is induced by oxidative stress and confers cytoprotection to limit tissue damage (36), was unchanged in UCP2BKO islets (Fig. 3C). These findings suggest that long-term elevation of ROS in UCP2BKO islets induces changes in signal transduction pathways that lead to altered antioxidant gene expression; however, these ROS levels are not high enough to induce oxidative stress/damage that requires cytoprotection by HO-1.
FIG. 3.
Higher intracellular ROS levels and antioxidant gene expression in UCP2BKO islets. A: Fluorescent microscopy was used to image intracellular H2O2 levels in islets isolated from RIPCre and UCP2BKO mice that were cultured overnight and incubated with the ROS-sensitive fluorescent dye CM-H2-DCFDA, in the presence of 11 mmol/L glucose. Islet intracellular H2O2 levels were manipulated by incubation of RIPCre islets with (+) or without (−) the pro-oxidant DEM (5 mmol/L) or UCP2BKO islets with (+) or without (−) the antioxidant NAC (0.2 mmol/L). Data shown are expressed as the fold-change over RIPCre islets. Representative fluorescent microscopy images of each condition are shown above each bar. n = 11–16 islets from 4 mice/genotype. *P < 0.05, ***P < 0.0001. B: Measurement of intracellular H2O2 in β-cells selected from dispersed islet cells. H2O2 was measured as in A. A total of 20–30 larger cells (i.e. β-cells) were selected for each coverslip. n = 5–7. C: Quantitative PCR was used to quantify the expression of several antioxidant genes as well as the oxidative stress-responsive HO-1 in isolated islets. Data shown are expressed as a percentage of β-actin mRNA expression levels for n = 3–7. *P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)
UCP2 deficiency in β-cells enhances GSIS in vitro in a ROS-dependent manner.
To investigate the effect of β-cell–specific UCP2 deletion on insulin secretion, GSIS was measured in RIPCre and UCP2BKO isolated islets using an islet perifusion system (Fig. 4A and B). At 2.8 mmol/L glucose, RIPCre and UCP2BKO secreted similar amounts of insulin. At 11 mmol/L glucose, insulin secretion was more rapid and robust in the UCP2BKO islets (Fig. 4B). Similarly, insulin secretion was greater in UCP2BKO islets compared with RIPCre controls at 16.7 mmol/L glucose (Fig. 4C), demonstrating that UCP2 deficiency in β-cells enhances GSIS but has no effect on insulin secretion at low glucose concentrations. In addition, preincubation of human islets with genipin, a known UCP2 inhibitor (37), resulted in significantly enhanced GSIS (Fig. 4F), further proof that acute inhibition of UCP2 enhances GSIS. To our knowledge, this is the first demonstration that the role of UCP2 in mouse islets is shared with human islets.
FIG. 4.

UCP2BKO islets display ROS-dependent enhanced GSIS. A: Measurement of GSIS over time in RIPCre and UCP2BKO islets by perifusion with varying concentrations of glucose (2.8, 11, and 16.7 mmol/L). Areas under the curve (AUC) are shown for 11 mmol/L glucose (B) and 16.7 mmol/L glucose (C). n = 6. *P < 0.05. Measurement of GSIS after manipulation of intracellular ROS levels in RIPCre (D) and UCP2BKO (E) islets using the pro-oxidant DEM (5 mmol/L) or the antioxidant NAC (0.2 mmol/L) by static incubation. Islets were preincubated in 2.8 mmol/L glucose for 1 h (±5 mmol/L DEM or 0.2 mmol/L NAC), followed by 30-min incubation in 2.8 or 16.7 mmol/L glucose (±5 mmol/L DEM or 0.2 mmol/L NAC). Insulin secretion was measured in nanograms of insulin per islet. n = 4. *P < 0.05. F: Measurement of GSIS in human islets in the presence or absence of 50 µmol/L genipin. Human islets were subjected to a similar static secretion protocol as mouse islets. Acute preincubation in genipin, a known UCP2 inhibitor, stimulates insulin secretion from human islets. n = 3 independent experiments. **P < 0.01. LG, low glucose; HG, high glucose. The error bars show the SEM.
Recent studies suggest that exposure to moderately elevated ROS stimulates insulin secretion in vitro (16–18), therefore the effect of manipulating ROS levels on insulin secretion was investigated. Preincubation of UCP2BKO islets with 0.2 mmol/L NAC, which promotes H2O2 scavenging by increasing glutathione synthesis, reduced intracellular H2O2 levels to a level similar to RIPCre islets (Fig. 3A) and consequently reduced GSIS (Fig. 4E). Conversely, pretreatment of RIPCre islets with 5 mmol/L DEM, which promotes ROS formation through interference with glutathione synthesis, boosted intracellular H2O2 levels (Fig. 3A) and resulted in escalated GSIS (Fig. 4D). Therefore, elevated intracellular ROS is at least one contributing factor to the elevated GSIS observed in UCP2-deficient β-cells.
UCP2 deficiency in β-cells is associated with greater α-cell area, increased islet glucagon content, and aberrantly increased glucose-induced glucagon secretion.
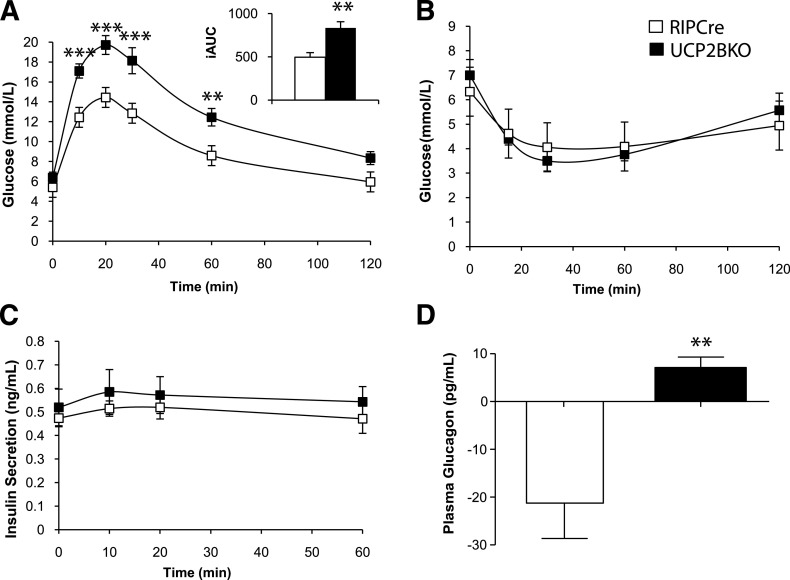
Despite having enhanced GSIS in vitro (Fig. 4), oral glucose tolerance tests (OGTTs) revealed that UCP2BKO mice are glucose-intolerant compared with RIPCre mice (Fig. 5A). No differences in body weight, fasting plasma insulin, and glucagon levels or in daily fasting blood glucose were observed between the two genotypes (Supplementary Fig. 2). Insulin tolerance tests (ITTs) revealed no differences in insulin sensitivity (Fig. 5B). Plasma insulin levels during the OGTT were also similar in both genotypes (Fig. 5C); however, plasma glucagon levels measured 10 min after gavage (just before the peak of blood glucose) were suppressed in RIPCre mice but were aberrantly increased in UCP2BKO mice (Fig. 5D). Since islets are anatomically complex microorgans consisting of a heterogeneous population of endocrine cells (all of which can significantly affect glucose homeostasis), the effect of β-cell UCP2-deficiency on the function and morphology of α-cells was investigated. No difference in the number of islets per pancreatic area was observed (Fig. 6A). Insulin-positive areas in UCP2BKO and RIPCre pancreata (Fig. 6B) were similar, suggesting equal β-cell masses. Conversely, UCP2BKO pancreata had a greater glucagon-positive area compared with RIPCre islets (Fig. 6C), indicative of increased α-cell mass. Total islet insulin content (Fig. 7B) was similar between the two genotypes; however, glucagon content was significantly elevated in UCP2BKO islets (Fig. 7A). Overall, UCP2 deficiency in β-cells induced increased α-cell mass and total islet glucagon content.
FIG. 5.
UCP2BKO mice are glucose intolerant. A: OGTTs in 12-week-old mice after an overnight fast. The inset shows the incremental area under the glucose curve (iAUC) B: ipITT in 13-week-old mice after a 4-h fast. C: Plasma insulin levels during the OGTT. D: The change in plasma glucagon levels during the first 10 min of the OGTT. Blood samples were taken at 0 and 10 min after glucose gavage, and the change in plasma glucagon level over 10 min was calculated. n = 8–15 mice per genotype. **P < 0.01, ***P < 0.001. The error bars show the SEM.
FIG. 6.
Islet morphology. UCPBKO islets have increased α-cell mass. A: Representative images of sectioned and stained mouse pancreata (original magnification ×40). Sections were quantified for islet number per area (mm2). B: Representative images of sectioned and insulin-stained mouse pancreata (original magnification ×400). The insulin-positive area was calculated and normalized to pancreatic slice area. C: Representative images of sectioned and glucagon-stained mouse pancreata (original magnification ×400). The glucagon-positive area was calculated and normalized to pancreatic slice area. n = 4–5 mice/genotype. *P < 0.05. The error bars show the SEM. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 7.
Total glucagon content and glucagon secretion are augmented in UCP2BKO islets. A: Total glucagon content of isolated RIPCre and UCP2BKO islets. Data for n = 6. *P < 0.05. B: Total insulin content of isolated RIPCre and UCP2BKO islets. n = 4–6. C: Glucagon secretion from isolated RIPCre and UCP2BKO islets after incubation in 16.7 mmol/L glucose with (+) or without (−) DEM or NAC, respectively, for 1 h. n = 3–4. *P < 0.05; **P < 0.01. The error bars show the SEM.
Furthermore, the effect of ROS on glucagon secretion in vitro was investigated under high glucose (16.7 mmol/L) conditions when glucagon secretion is normally blunted. Untreated UCP2BKO islets secrete approximately 4.5-fold more glucagon than RIPCre islets in high glucose conditions (Fig. 7C), consistent with our in vivo measurements. Preincubation of UCP2BKO islets with antioxidant (NAC) reduced both intracellular ROS levels (Fig. 3A) and glucagon secretion (Fig. 7C). Similarly, pretreatment of RIPCre islets with pro-oxidant (DEM) increased intracellular ROS levels (Fig. 3A) and glucagon secretion (Fig. 7C). Overall, higher intracellular ROS levels are associated with and appear to promote aberrant glucagon secretion when islets are exposed to a high glucose concentration. This suggests that UCP2 deficiency in the β-cell promotes glucagon secretion in a ROS-dependent manner, and therefore, β-cell UCP2 plays a role in the regulation of intraislet ROS signals that regulate α-cell function and morphology.
DISCUSSION
UCP2 function in the β-cell.
Using a novel β-cell–specific UCP2KO mouse model, we have been able to shed some light on a long-debated issue regarding UCP2 function in primary β-cells. UCP2 deficiency in the β-cell is associated with higher rates of total respiration and greater glucose-induced ΔΨm, without increased mitochondrial coupling efficiency or altered ATP levels. Therefore, by definition, UCP2 does not function as a classical uncoupling protein in primary β-cells.
Significant evidence exists for a role of UCP2 in the regulation of ROS production (18,20,27,28), which has only been tested in UCP2KO models and clonal cell lines. Intracellular ROS is significantly elevated in UCP2BKO islets and β-cells, strengthening this connection between ROS regulation and UCP2. Given that mitochondrial coupling is unchanged, increased ROS formation in UCP2BKO islets is not the result of reduced proton leak in the inner mitochondrial membrane. Rather, UCP2BKO β-cells display mildly increased glucose-induced ΔΨm, which is one potential consequence of greater OCR in these islets and can lend to greater mitochondrial ROS formation. Mitochondrial ROS formation occurs very efficiently at higher ΔΨm (38). Thus, even a mild increase in mitochondrial membrane hyperpolarization can significantly promote mitochondrial ROS formation. Alternatively, other undiscovered or unconfirmed transport functions of UCP2 in the β-cell may indirectly contribute to the regulation of mitochondrial ROS formation and/or the control of glucose-induced mitochondrial membrane hyperpolarization.
Elevated intracellular H2O2 levels in UCP2BKO islets consequently correlated with enhanced GSIS, confirming that ROS are important signaling molecules that regulate GSIS (16–18). Although we did not investigate the specific targets of ROS signaling that control GSIS in this study, we did demonstrate that ROS alone is not sufficient to promote insulin secretion in the absence of high glucose. That is, basal insulin secretion was not higher in UCP2BKO islets that had higher intracellular ROS; moreover, pro-oxidant treatment of RIPCre islets did not stimulate insulin secretion when incubated in low glucose. These findings conflict those of Pi et al. (2007) (17), who demonstrated that ROS alone sufficiently promoted insulin secretion independently of glucose concentration in INS-1E cells and isolated mouse islets; however, in a separate set of experiments, they demonstrated that inhibition of mitochondrial ATP production by mitochondrial electron transport chain inhibitors, which also increase ROS, subsequently inhibited GSIS. This suggests that without ATP stimulation, GSIS cannot be amplified, despite increased ROS levels (17). A similar phenomenon was demonstrated in the 2009 study of Leloup et al. (16). Therefore, ROS alone is not a suitable “trigger” for insulin secretion, but is more likely an “amplifying” signal (39). The ATP-dependent triggering pathway of insulin secretion has been well characterized; however, the amplifying pathway of GSIS is less well defined. It is, at least in part, thought to result from increased efficacy of calcium on the exocytotic machinery (39). Interestingly, it has been shown that calcium mobilization during GSIS is ROS-dependent (16,17).
β-Cell UCP2 and the α-cell.
Previous studies on whole-body UCP2KO mice revealed improved glucose tolerance (25) or no change in glucose tolerance (28) after an OGTT. Unexpectedly, UCP2BKO mice were glucose-intolerant. Both genotypes had similar insulin sensitivities and plasma insulin levels after glucose challenge, which suggests that impaired glucose tolerance in the UCP2BKO mouse is not due to impaired glucose clearance but to rather some other dysfunction of glucose homeostasis. Importantly, islets are anatomically complex and sophisticated microorgans consisting of a heterogeneous population of endocrine cells, all of which can significantly affect glucose homeostasis. For this reason, the effect of β-cell UCP2-deficiency on the function and morphology of pancreatic α-cells was investigated.
UCP2 deletion in β-cells resulted in greater islet glucagon content and atypically increased glucagon secretion (both in vitro and in vivo) under high glucose conditions. This finding differs dramatically from the whole-body UCP2KO mice, which showed impaired glucagon secretion (25). Unlike in the UCP2KO animals, UCP2BKO α-cells retain UCP2 expression, which may be a contributing factor to the heightened glucagon secretion observed under high glucose conditions, although this remains to be proven. Importantly, the abnormal glucagon secretion observed under high glucose conditions in UCP2BKO islets was ROS-dependent. Evidently, ROS generated in the β-cell as a consequence of UCP2 deletion potentially functions as an intraislet signal that regulates α-cell function. As such, β-cell UCP2 is important not only in the regulation of ROS signals that regulate GSIS and β-cell function but also in the control of intraislet ROS signals that contribute to the regulation of α-cell function. If we relate this finding back to the observed glucose intolerance of the UCP2BKO mice, aberrant glucagon secretion under high glucose conditions could provide one potential explanation for this phenotype; however, it is important to acknowledge that several other potentially complex factors could also contribute to the glucose intolerant phenotype of these mice.
Recently, a small population of hypothalamic neurons were shown to express Cre recombinase under the rat insulin promoter in transgenic mice (40). UCP2 is expressed in the hypothalamus, yielding the potential for UCP2 deletion in the brain. Although the identity and function of these RIPCre-expressing hypothalamic neurons remains unknown, we cannot deny their potential contribution to glucose homeostasis. Future studies, including measurement of hepatic gluconeogenesis and/or peripheral insulin sensitivity by the clamp protocol, could provide interesting insight into the glucose-intolerant phenotype of the UCP2BKO mouse.
In addition to altered α-cell function, α-cell mass was greater in UCP2BKO pancreata, which could be a direct consequence of higher islet ROS concentrations, because moderate increases in ROS have been shown to directly stimulate cell proliferation in many cell types (41). Interestingly, increasing concentrations of insulin (one consequence of UCP2 deficiency in β-cells) can also increase α-cell proliferation through the insulin-receptor signaling pathway (42). Additionally, ROS have been shown to further promote insulin action via ROS-dependent inactivation of protein tyrosine phosphatases (43). We can therefore speculate that greater insulin secretion and elevated ROS levels, at least in part, contribute to the increased α-cell mass seen in the UCP2BKO pancreata.
Despite the well-established paracrine inhibitory effect of insulin on glucagon secretion (44,45), we observed a ROS-dependent escalation of glucagon secretion in the presence of elevated GSIS, suggesting that ROS-mediated regulation of glucagon secretion occurs independently of insulin action. Although the mechanism(s) and targets of ROS-mediated regulation of α-cell function are largely unknown, this type of regulation of α-cell function may be a significant player in the development of hyperglycemia and type 2 diabetes. Clinical studies have revealed that lack of suppression of postprandial glucagon secretion significantly contributes to hyperglycemia observed in type 2 diabetic patients (46–48), conditions that are also associated with elevated levels of ROS (20,49).
Our findings using the UCP2BKO mouse model have revealed that UCP2 does not behave as a classical uncoupler in the pancreatic β-cell. It appears that at least one function of β-cell UCP2 is to control intracellular and intraislet ROS signals, which in turn regulate β- and α-cell function, respectively; however, the precise contribution of UCP2 to regulation of both intracellular and intraislet ROS signals under various physiologically relevant conditions is unclear. We know that ROS levels increase dramatically during chronic hyperglycemia (20,49), hyperlipidemia (23,50), and in diabetic mouse models (20,49), and that UCP2 activity increases under similar conditions (19,20), potentially to combat oxidative stress and stave-off cytotoxicity; however, UCP2 function may differ dramatically in a healthy cell and may have a more subtle role in the regulation of GSIS-amplifying ROS signals. Therefore, it will be imperative to clearly identify the conditions of UCP2 activation and deactivation and contribution to ROS signaling in both healthy and diseased states to fully elucidate UCP2 function in β-cells.
ACKNOWLEDGMENTS
This study was funded by a Canadian Institutes for Health Research (CIHR) operating grant (MOP 12898) to M.B.W., National Institutes of Health grants R01 DK074778 and R01 DK56690-11 to O.S.S., the Boston University Mitochondria Affinity Research Collaborative (mtARC), a Banting & Best Diabetes Centre Novo Nordisk studentship to S.S., a Canadian Diabetes Association postdoctoral fellowship award to E.M.A., and a CIHR postdoctoral fellowship award to C.A.R.-D.
No potential conflicts of interest relevant to this article were reported.
C.A.R.-D. researched data, contributed to experimental design, contributed to discussion, and wrote and edited the manuscript. S.S. researched data, contributed to experimental design, contributed to discussion, and reviewed the manuscript. E.M.A. researched data, contributed to experimental design, contributed to discussion, and edited and reviewed the manuscript. A.B., V.K., and K.J.P. researched data. J.D.W. and S.B.S. researched data, contributed to experimental design, and reviewed the manuscript. M.B.W. and O.S.S. contributed to experimental design, contributed to discussion, and edited and reviewed the manuscript.
We offer a special thanks to A.M.J. Shapiro and T. Kin at the Clinical Islet Laboratory, University of Alberta (Edmonton, AB, Canada) for the human islets used in this study and to Dr. Herbert Gaisano (Department of Physiology, University of Toronto) for assistance with perifusion studies.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0132/-/DC1.
C.A.R.-D., S.S., and E.M.A. contributed equally to this work.
REFERENCES
- 1.Fleury C, Neverova M, Collins S, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet 1997;15:269–272 [DOI] [PubMed] [Google Scholar]
- 2.Echtay KS. Mitochondrial uncoupling proteins—what is their physiological role? Free Radic Biol Med 2007;43:1351–1371 [DOI] [PubMed] [Google Scholar]
- 3.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2005;2:85–93 [DOI] [PubMed] [Google Scholar]
- 4.Echtay KS, Roussel D, St-Pierre J, et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002;415:96–99 [DOI] [PubMed] [Google Scholar]
- 5.Echtay KS, Esteves TC, Pakay JL, et al. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J 2003;22:4103–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker N, Vidal-Puig A, Brand MD. Stimulation of mitochondrial proton conductance by hydroxynonenal requires a high membrane potential. Biosci Rep 2008;28:83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couplan E, del Mar Gonzalez-Barroso M, Alves-Guerra MC, Ricquier D, Goubern M, Bouillaud F. No evidence for a basal, retinoic, or superoxide-induced uncoupling activity of the uncoupling protein 2 present in spleen or lung mitochondria. J Biol Chem 2002;277:26268–26275 [DOI] [PubMed] [Google Scholar]
- 8.Nedergaard J, Cannon B. The ‘novel’ ‘uncoupling’ proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp Physiol 2003;88:65–84 [DOI] [PubMed] [Google Scholar]
- 9.Bouillaud F. UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochim Biophys Acta 2009;1787:377–383 [DOI] [PubMed] [Google Scholar]
- 10.Arsenijevic D, Onuma H, Pecqueur C, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 2000;26:435–439 [DOI] [PubMed] [Google Scholar]
- 11.Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic β-cell function. Diabetes Obes Metab 2010;12(Suppl. 2):141–148 [DOI] [PubMed] [Google Scholar]
- 12.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003;52:1–8 [DOI] [PubMed] [Google Scholar]
- 13.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47–95 [DOI] [PubMed] [Google Scholar]
- 14.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997;46:1733–1742 [DOI] [PubMed] [Google Scholar]
- 15.Jezek P, Zácková M, Růzicka M, Skobisová E, Jabůrek M. Mitochondrial uncoupling proteins—facts and fantasies. Physiol Res 2004;53(Suppl. 1):S199–S211 [PubMed] [Google Scholar]
- 16.Leloup C, Tourrel-Cuzin C, Magnan C, et al. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 2009;58:673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pi J, Bai Y, Zhang Q, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007;56:1783–1791 [DOI] [PubMed] [Google Scholar]
- 18.Affourtit C, Jastroch M, Brand MD. Uncoupling protein-2 attenuates glucose-stimulated insulin secretion in INS-1E insulinoma cells by lowering mitochondrial reactive oxygen species. Free Radic Biol Med 2011;50:609– 616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patanè G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM. Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-gamma inhibition. Diabetes 2002;51:2749–2756 [DOI] [PubMed] [Google Scholar]
- 20.Krauss S, Zhang CY, Scorrano L, et al. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest 2003;112:1831–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan CB, De Leo D, Joseph JW, et al. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes 2001;50:1302–1310 [DOI] [PubMed] [Google Scholar]
- 22.Lameloise N, Muzzin P, Prentki M, Assimacopoulos-Jeannet F. Uncoupling protein 2: a possible link between fatty acid excess and impaired glucose-induced insulin secretion? Diabetes 2001;50:803–809 [DOI] [PubMed] [Google Scholar]
- 23.Joseph JW, Koshkin V, Zhang CY, et al. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes 2002;51:3211–3219 [DOI] [PubMed] [Google Scholar]
- 24.González-Barroso MM, Giurgea I, Bouillaud F, et al. Mutations in UCP2 in congenital hyperinsulinism reveal a role for regulation of insulin secretion. PLoS One 2008;3:e3850. 10.1371/journal.pone.0003850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 2001;105:745–755 [DOI] [PubMed] [Google Scholar]
- 26.Joseph JW, Koshkin V, Saleh MC, et al. Free fatty acid-induced beta-cell defects are dependent on uncoupling protein 2 expression. J Biol Chem 2004;279:51049–51056 [DOI] [PubMed] [Google Scholar]
- 27.Lee SC, Robson-Doucette CA, Wheeler MB. Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin. J Endocrinol 2009;203:33–43 [DOI] [PubMed] [Google Scholar]
- 28.Pi J, Bai Y, Daniel KW, et al. Persistent oxidative stress due to absence of uncoupling protein 2 associated with impaired pancreatic beta-cell function. Endocrinology 2009;150:3040–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diao J, Allister EM, Koshkin V, et al. UCP2 is highly expressed in pancreatic alpha-cells and influences secretion and survival. Proc Natl Acad Sci USA 2008;105:12057–12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pecqueur C, Alves-Guerra MC, Gelly C, et al. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem 2001;276:8705–8712 [DOI] [PubMed] [Google Scholar]
- 31.Parton LE, Ye CP, Coppari R, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 2007;449:228–232 [DOI] [PubMed] [Google Scholar]
- 32.Kong D, Vong L, Parton LE, et al. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab 2010;12:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gyulkhandanyan AV, Lu H, Lee SC, et al. Investigation of transport mechanisms and regulation of intracellular Zn2+ in pancreatic alpha-cells. J Biol Chem 2008;283:10184–10197 [DOI] [PubMed] [Google Scholar]
- 34.Hardy AB, Fox JE, Giglou PR, et al. Characterization of Erg K+ channels in alpha- and beta-cells of mouse and human islets. J Biol Chem 2009;284:30441–30452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asghar Z, Yau D, Chan F, Leroith D, Chan CB, Wheeler MB. Insulin resistance causes increased beta-cell mass but defective glucose-stimulated insulin secretion in a murine model of type 2 diabetes. Diabetologia 2006;49:90–99 [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi G, Yoshida T, Noguchi M. Heme oxygenase and heme degradation. Biochem Biophys Res Commun 2005;338:558–567 [DOI] [PubMed] [Google Scholar]
- 37.Zhang CY, Parton LE, Ye CP, et al. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab 2006;3:417–427 [DOI] [PubMed] [Google Scholar]
- 38.Nègre-Salvayre A, Hirtz C, Carrera G, et al. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J 1997;11:809–815 [PubMed] [Google Scholar]
- 39.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000;49:1751–1760 [DOI] [PubMed] [Google Scholar]
- 40.Wicksteed B, Brissova M, Yan W, et al. Conditional gene targeting in mouse pancreatic ß-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 2010;59:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiarugi P, Fiaschi T. Redox signalling in anchorage-dependent cell growth. Cell Signal 2007;19:672–682 [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Kim W, Chen Z, et al. Insulin and glucagon regulate pancreatic α-cell proliferation. PLoS One 2011;6:e16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes 2005;54:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diao J, Asghar Z, Chan CB, Wheeler MB. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic alpha-cells. J Biol Chem 2005;280:33487–33496 [DOI] [PubMed] [Google Scholar]
- 45.Kawamori D, Kurpad AJ, Hu J, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab 2009;9:350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975;1:14–16 [DOI] [PubMed] [Google Scholar]
- 47.Dobbs R, Sakurai H, Sasaki H, et al. Glucagon: role in the hyperglycemia of diabetes mellitus. Science 1975;187:544–547 [DOI] [PubMed] [Google Scholar]
- 48.Sakurai H, Dobbs RE, Unger RH. The role of glucagon in the pathogenesis of the endogenous hyperglycemia of diabetes mellitus. Metabolism 1975;24:1287–1297 [DOI] [PubMed] [Google Scholar]
- 49.Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem 2003;278:9796–9801 [DOI] [PubMed] [Google Scholar]
- 50.Carlsson C, Borg LA, Welsh N. Sodium palmitate induces partial mitochondrial uncoupling and reactive oxygen species in rat pancreatic islets in vitro. Endocrinology 1999;140:3422–3428 [DOI] [PubMed] [Google Scholar]