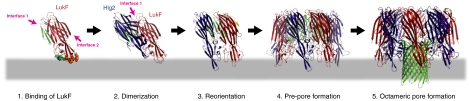
Fig. 4.
Mechanism of assembly for staphylococcal γ-HL. (1) Binding of LukF. LukF binds to the erythrocyte surface via Tyr72, Trp257, Phe260, and Tyr 261 (shown as orange spheres) inclined with respect to its molecular axis. Trp177 and Arg198 (shown as green spheres) capture lipid head groups. As a consequence of the orientation, interface 1 is exposed to the surface, whereas interface 2 is hindered. The membrane is shown as a gray bar. (2) Dimerization. Hlg2 binds to LukF through the surface-exposed interface 1. Upon binding, the amino-latch of Hlg2 is released from the β-sheet due to the structural clash with loop A (LukF). At this step, the prestem is not released completely from the cap domain. (3) Reorientation. The heterodimer changes the orientation to expose interface 2. Binding of Hlg2 with the proteinaceous component(s) may induce this motion. (4) Prepore formation. The heterodimer assembles into a prepore. Upon octameric assembly, the long amino-latch of LukF [yellow in (3)] is released from the cap domain. The electrostatic interactions between loop A and β-1 are formed completely in both interfaces, which induces a conformation change of the prestem into the partially unfolded state and release of the prestem from the cap domain (green loops). (5) Octameric pore formation. The prestem completely unfolds and all hydrogen bonds are disrupted. Unfolded prepore penetrates into the membrane and forms a transmembrane β-barrel (green cartoon).