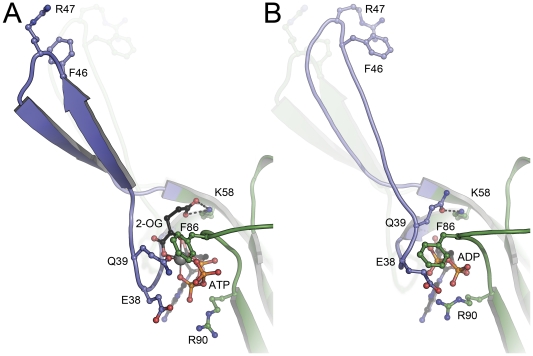
Figure 2. Structural differences between (A) the ATP:Mg2+:2-OG complex and (B) the ADP complex of Af-GlnK3.
In (A) the key ligand 2-oxolutarate requires the presence of ATP for binding and is located at the base of the T-loop (blue), with its ã-carboxy group forming a hydrogen bond to the conserved K58. Residue Q39 is the only protein ligand to the Mg2+ ion (grey sphere), and it is this residue that in the ADP complex (B) attains the exact position of 2-OG in (A), forming an analogous hydrogen bond to K58. The resulting tilt and shift of the base of the T-loop leads to a stable â-hairpin structure in (A), compared to a less well-ordered loop in (B) that moves inward by 20°towards the trimer. In both structures, the respective other T-loop conformation is indicated.