Abstract
Background
The purpose of this paper is to report on the bacterial species isolated from patients with bacterial conjunctivitis participating in three clinical trials of besifloxacin ophthalmic suspension, 0.6%, and their in vitro antibacterial susceptibility profiles.
Methods
Microbial data from three clinical studies, conducted at multiple clinical sites in the US and Asia were integrated. Species were identified at a central laboratory, and minimum inhibitory concentrations were determined for various antibiotics, including β-lactams, fluoroquinolones, and macrolides.
Results
A total of 1324 bacterial pathogens representing more than 70 species were isolated. The most common species were Haemophilus influenzae (26.0%), Streptococcus pneumoniae (22.8%), Staphylococcus aureus (14.4%), and Staphylococcus epidermidis (8.4%). H. influenzae was most frequently isolated among patients aged 1–18 years, while S. aureus was most prevalent among those >65 years. Drug resistance was prevalent: Of H. influenzae isolates, 25.3% were β-lactamase positive and 27.2% of S. pneumoniae isolates were penicillin-intermediate/ resistant; of S. aureus isolates, 13.7% were methicillin-resistant (MRSA), and of these, 65.4% were ciprofloxacin-resistant, while 45.9% of S. epidermidis isolates were methicillin-resistant (MRSE), and, of these, 47.1% were ciprofloxacin-resistant. Besifloxacin was more potent than comparator fluoroquinolones overall, and particularly against Gram-positive bacteria. Against ciprofloxacin-resistant MRSA and MRSE, besifloxacin was four-fold to ≥ 128-fold more potent than other fluoroquinolones.
Conclusions
While the pathogen distribution in bacterial conjunctivitis has not changed, drug resistance is increasing. Patient age and local antibiotic resistance trends should be considered in the treatment of this ocular infection. Besifloxacin showed broad-spectrum in vitro activity and was particularly potent against multidrug-resistant staphylococcal isolates.
Keywords: besifloxacin, fluoroquinolones, in vitro activity, ocular isolates, bacterial conjunctivitis
Introduction
Conjunctivitis is an inflammation of the thin, transparent mucous membrane covering the eye.1 Bacterial conjunctivitis is a common external ocular infection that affects persons of all ages.2,3 Although acute conjunctivitis can be viral in nature, the majority of cases in children and approximately 50% of cases in adults are caused by bacteria.2,4 Some of the more common causative organisms can be components of the normal eyelid flora (eg, Staphylococcus aureus, Staphylococcus epidermidis) or nasopharyngeal flora (eg, Haemophilus influenzae, Streptococcus pneumoniae).1,2,5–7 Other common pathogens that can cause bacterial conjunctivitis include Moraxella spp, Neisseria spp, Corynebacterium spp, and other Streptococcus spp.2
Besifloxacin, an N-1 cyclopropyl, 8-chloro-fluoroquinolone, was developed for the topical treatment of ocular infections. Besifloxacin demonstrates potent inhibition of both bacterial DNA gyrase and topoisomerase IV noted for some fluoroquinolones. Cambeau et al found besifloxacin to be as active against the DNA gyrase of S. pneumoniae as against topoisomerase IV.8 In vitro studies showed the new fluoroquinolone to be effective against both Gram-positive and Gram-negative bacteria, as well as multidrug-resistant strains9 and to be rapidly bactericidal for the common pathogens of bacterial conjunctivitis.10,11
Besifloxacin ophthalmic suspension 0.6% was approved in 2009 by the US Food and Drug Administration for the treatment of bacterial conjunctivitis. Three clinical trials were conducted to evaluate the clinical and microbiological efficacy of besifloxacin ophthalmic suspension 0.6% compared with vehicle, or moxifloxacin ophthalmic solution 0.5% dosed three times daily for 5 days.12–14 Integrated clinical microbiological eradication rates for the three studies are described in the companion paper by Morris et al.15 Here we describe those bacterial pathogens most commonly isolated from patients in these studies and their in vitro antibacterial susceptibility to besifloxacin, comparator fluoroquinolones, and other ophthalmic antibacterial drugs. Pathogen distribution was further characterized by the age of the patient and by geography.
Methods
Studies
Microbiological data for bacterial isolates from three prospective, randomized, multicenter, double-masked clinical trials (two vehicle-controlled and one active-controlled) evaluating the clinical safety and efficacy of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis were integrated. A general description of the trial designs is presented in the companion manuscript by Morris et al.15 Individual study results have been reported previously.12–14
In each study, microbiological cultures of the conjunctival cul-de-sac were taken at visit 1 (day 1), visit 2 (day 4 ± 1 or day 5 ± 1),12–14 and visit 3 (day 8 or 9) prior to administration of the morning treatment dose. Microbiological samples were collected on a sterile swab and inserted into validated transport medium. The collection procedure was repeated with a second swab for viral culture using viral transport medium. All swabs were transported under refrigerated or frozen conditions to a central laboratory (Covance Central Laboratory Services, Indianapolis, IN) for analysis. Briefly, 0.5 mL and 0.1 mL aliquots of the suspended specimen were inoculated onto chocolate agar and trypticase soy agar with 5% sheep blood. Two serial 10-fold dilutions were also inoculated onto separate plates of each medium for quantitative plate counts. All inoculated plates were incubated at 35°C in 5%–7% CO2 atmosphere, and bacterial colonies were counted after 24 and 48 hours of incubation. A specimen sample (0.1 mL) and two 10-fold dilutions for yeast culture were inoculated onto Sabouraud dextrose agar and incubated at 30°C. Yeast/mycelial colonies were counted after a total of 48–120 hours (2–5 days) of incubation. For viral culture, a 0.2 mL aliquot of specimen suspended in transport medium was inoculated into appropriate cell lines, incubated for 1 hour at 36°C, then washed, refed with maintenance media, and observed daily for 2–3 weeks for cytopathic effect. Commercial immunofluorescent reagents were used to identify adenovirus and herpes simplex virus.
Bacterial isolates were considered causative of the conjunctivitis if the colony count equaled or exceeded the threshold value on the Cagle list as modified by Leibowitz.16,17
In vitro susceptibility testing
In vitro susceptibilities to antibacterial agents were determined for all bacterial isolates at or above the Cagle threshold at baseline.16,17 Antibacterial agents evaluated included besifloxacin, moxifloxacin, azithromycin, ciprofloxacin, gatifloxacin, levofloxacin, and ofloxacin. Penicillin antimicrobial susceptibilities were determined for S. pneumoniae and beta-hemolytic streptococci, and oxacillin susceptibilities were determined for all Staphylococcus species. Susceptibility testing was conducted on microtiter plates manufactured by Covance Central Laboratory. Minimum inhibitory concentrations (MICs) were determined by broth microdilution according to the procedure recommended by the Clinical and Laboratory Standards Institute (CLSI).18,19 Isolates from selected species were further characterized by their antimicrobial resistance phenotype. Ciprofloxacin was chosen to determine sensitivity or resistance to fluoroquinolones. H. influenzae isolates were classified as β-lactamase positive or negative. Strains of S. aureus and S. epidermidis were designated methicillin-sensitive or methicillin-resistant based on current CLSI breakpoints for oxacillin.20,21 Similarly, S. pneumoniae isolates were designated as being penicillin-sensitive, penicillin-intermediate, or penicillin-resistant based on CLSI breakpoints for oral penicillin.20,21 As an exclusively topical agent, besifloxacin susceptibility test interpretive criteria (ie, breakpoints) have not been established; however, quality control ranges for besifloxacin susceptibility testing were included.22 Initial fluoroquinolone MIC testing spanned a drug concentration range of 0.004–8 μg/mL. All besifloxacin MICs were within the initial test drug concentration range (≤8 μg/mL). Because initial MIC testing of several S. aureus and S. epidermidis isolates resulted in at least one fluoroquinolone comparator MIC exceeding the highest concentration (8 μg/mL) tested, these isolates were retested in triplicate with a higher range of drug concentrations for all fluoroquinolones (up to 512 μg/mL) to obtain endpoint values for all fluoroquinolones.
Integrated analyses
Microbiological data from all three studies were pooled for a comprehensive, integrated analysis. The proportions of individual species at or above threshold across the three studies were tabulated along with their in vitro susceptibilities and antimicrobial resistance phenotypes. While only one eye per patient (study eye) was considered for the primary efficacy endpoints of clinical resolution and bacterial eradication in the clinical study analyses,12–14 both eyes could contribute microbiological samples to the integrated analyses if both eyes had signs and symptoms of bacterial conjunctivitis and the pathogenic organism in the nonstudy eye was different from the organism in the study eye. In addition, more than one species from each eye was included if each species met the Cagle criteria.
Results
Pathogen distribution
A total of 1324 bacterial pathogens were isolated at baseline (visit 1) at or above the Cagle threshold from 1041 culture-confirmed bacterial conjunctivitis patients across the three clinical studies, with 92.8% (1229/1324) and 7.2% (95/1324) of the bacterial isolates obtained from patients at US and Asian clinical sites, respectively. Although some isolates could only be identified to the genus or group level, more than 70 different species of bacteria were identified. Isolates identified included 430 streptococci, 349 Haemophilus spp, 342 staphylococci, 73 corynebacteria, 24 Enterobacteriaceae, 23 Moraxella spp, 9 Pseudomonas spp, and 7 Neisseria spp. In addition, 148 patients were positive for viral cultures at baseline. Of the viral positive cultures, 94.6% (140/148) were identified as adenovirus and 5.4% (8/148) were identified as herpes simplex virus; 28 of these viral pathogens were isolated from eyes that also yielded bacterial isolates at or above threshold, indicating that 2.7% (28/1041) of bacterial culture-positive eyes were coinfected with virus. Yeast was rarely recovered from subjects with bacterial conjunctivitis. The few fungal isolates recovered included Candida parapsilosis (n = 1) at baseline and Penicillium spp (n = 1), Candida glabrata (n = 1), and Saccharomyces cerevisiae (n = 1) at subsequent visits.
Table 1 presents a listing of bacterial species isolated across the three clinical studies in decreasing order of prevalence. Gram-positive and Gram-negative bacteria contributed 66.9% (886/1324) and 33.1% (438/1324) of the isolates, respectively, and the four most prevalent species, H. influenzae, S. pneumoniae, S. aureus, and S. epidermidis, together accounted for 71.5% (947/1324) of all isolates. The species distribution among isolates obtained from patients enrolled at Asian clinical sites was similar to that observed for patients enrolled at US sites (data not shown), with the exception of S. pneumoniae, which accounted for 3.2% (3/95) of isolates from Asian clinical sites, compared with 24.3% (299/1229) of isolates from US clinical sites.
Table 1.
Bacterial pathogens in order of decreasing prevalence
| Organism | n (%) | Organism | n (%) | Organism | n (%) |
|---|---|---|---|---|---|
| Haemophilus influenzae | 344 (26.0) | Stenotrophomonas maltophilia | 9 (0.7) | Streptococcus pyogenes | 5 (0.4) |
| Streptococcus pneumoniae | 302 (22.8) | Streptococcus salivarius | 9 (0.7) | Nonfermentative Gram-negative rod | 4 (0.3) |
| Staphylococcus aureus | 190 (14.4) | Brevibacterium spp | 8 (0.6) | Rothia mucilaginosa | 4 (0.3) |
| Staphylococcus epidermidis | 111 (8.4) | Corynebacterium pseudodiphtheriticum | 8 (0.6) | Staphylococcus caprae | 4 (0.3) |
| Streptococcus mitis groupa | 45 (3.4) | Corynebacterium striatum | 8 (0.6) | Streptococcus sanguinis | 4 (0.3) |
| CDC coryneform group G | 29 (2.2) | Pseudomonas aeruginosa | 8 (0.6) | Achromobacter xylosoxidans | 3 (0.2) |
| Streptococcus mitis | 20 (1.5) | Staphylococcus lugdunensis | 8 (0.6) | Acinetobacter calcoaceticus | 3 (0.2) |
| Streptococcus oralis | 18 (1.4) | Corynebacterium macginleyi | 6 (0.5) | Corynebacterium spp | 3 (0.2) |
| Streptococcus sppa | 13 (1.0) | Corynebacterium propinquum | 6 (0.5) | Granulicatella adiacens | 3 (0.2) |
| Moraxella catarrhalis | 12 (0.9) | Proteus mirabilis | 6 (0.5) | Neisseria meningitidis | 3 (0.2) |
| Aerococcus viridans | 10 (0.8) | Staphylococcus warneri | 6 (0.5) | Staphylococcus haemolyticus | 3 (0.2) |
| Moraxella lacunata | 9 (0.7) | Enterococcus faecalis | 5 (0.4) | Streptococcus anginosus | 3 (0.2) |
| Serratia marcescens | 9 (0.7) | Haemophilus parainfluenzae | 5 (0.4) | Viridans streptococci | 3 (0.2) |
| Staphylococcus hominis | 9 (0.7) | Staphylococcus capitis | 5 (0.4) |
Notes: Isolates that were identified to the species level were listed separately. Species and phenotypes with less than three isolates: Abiotrophia defectiva (n = 1); Acinetobacter johnsonii (1); Acinetobacter spp (1); Bacillus spp (1); Brevibacterium casei (1); Brevundimonas vesicularis (1); CDC coryneform group I1 (1); Citrobacter koseri (1); Coagulase-negative staphylococci (2); Corynebacterium afermentans (2); Corynebacterium amycolatum (1); Corynebacterium argentoratense (2); Corynebacterium auris (1); Corynebacterium jeikeium (2); Corynebacterium minutissimum (2); Corynebacterium urealyticum (2); Eikenella corrodens (1); Enterobacter cloacae (1); Enterobacter sakazakii (1); fermentative Gram-negative rod (1); Gemella morbillorum (2); Gemella spp (2); Kingella denitrificans (1); Klebsiella oxytoca (2); Klebsiella ozaenae (1); Kocuria kristinae (2); Leminorella spp (1); Micrococcus spp (2); Moraxella catarrhalis, β-lactamase negative (1); Moraxella nonliquefaciens (1); Moraxella spp (1); Morganella morganii (2); Neisseria gonorrhoeae (2); Neisseria sicca (1); Neisseria subflava (1); Pasteurella multocida (1); Pseudomonas fluorescens (1); Staphylococcus chromogenes (1); Staphylococcus intermedius (1); Staphylococcus xylosus (2); Streptococcus agalactiae (1); Streptococcus anginosus group (1); Streptococcus dysgalactiae (2); Streptococcus milleri group (1); Streptococcus parasanguinis (2); and Streptococcus thermophilus (1).
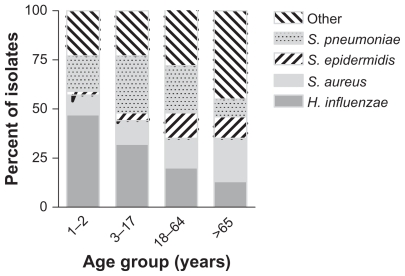
Of the isolates, 11.8% (156/1324) were contributed by patients aged 1–2 years, 32.3% (428/1324) by patients aged 3–18 years, 40.7% (539/1324) by patients aged 19–64 years, and 15.2% (201/1324) by patients aged 65 years and older. Figure 1 presents the pathogen distribution by age group. H. influenzae was the most prevalent species in patients aged 1–2 years, representing 46.8% (73/156) of the isolates in that age group, and gradually decreased in prevalence as the patient age increased. However, even in the patients aged 65 years and older, 13.4% (27/201) of all isolates were H. influenzae. S. pneumoniae was commonly isolated from patients 1–64 years of age and peaked with 29.7% (127/428) in the 3–18-year age group. Only 9.5% (19/201) of isolates in the 65+ age group were S. pneumoniae. Other species of Streptococcus, most notably those belonging to the Streptococcus mitis group, were also more prevalent in younger than in older patients (data not shown). Moraxella spp accounted for only 23 isolates, but those were more frequently isolated from patients aged 1–2 years and 3–18 years compared with older patients.
Figure 1.
Distribution of Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Staphylococcus epidermidis, and other species among bacterial conjunctivitis isolates stratified by age group.
In the older patient population, staphylococci and corynebacteria were most prevalent. S. aureus contributed 10.3% (16/156) of isolates to the youngest age group and increased in prevalence with the age of the population to 22.4% (45/201) of all isolates from patients 65 years and older. Similarly, unspeciated staphylococci and S. epidermidis increased from 0% to 1.9% (0/156 and 3/156, respectively) in 1–2-year-old patients to 8.5% to 11.0% (17/201 and 22/201) in the oldest patient group. The same trend was noted for corynebacteria, which increased in frequency with age from 1.3% (2/156) to 13.4% (27/201). The number of Enterobacteriaceae also increased with patient age, ranging from 0.3% (2/584) for patients 18 years or younger, to 2.6% (14/539) to 4.0% (8/201) for patients aged 19–64 years and 65 years and older, respectively. Age-specific isolation patterns were also observed for Moraxella spp, but the number of isolates was too small to draw any conclusions.
Overall in vitro susceptibility
Table 2 presents the MIC values for besifloxacin and comparator antibacterial agents for those species with ≥10 isolates recovered for all Gram-positive and all Gram-negative bacteria and for bacteria overall. For all 1324 isolates, the MIC50 and MIC90 for besifloxacin were 0.06 and 0.25 μg/mL, respectively. These values were lower than those of the other fluoroquinolones, where the comparable MIC50 values were in the range of 0.125–0.5 μg/mL, and the MIC90 values were in the range of 0.5–2 μg/mL. MIC50 and MIC90 values for Gram-positive bacteria were widely spread, showing a 16-fold difference between the most and least potent fluoroquinolone. Besifloxacin was the most potent agent in this group, followed by moxifloxacin, gatifloxacin, levofloxacin, ciprofloxacin, and ofloxacin. Against Gram-negative bacteria, values for the five fluoroquinolones varied only by a two-fold dilution for the MIC50 and a four-fold dilution for the MIC90. Against these organisms, the older fluoroquinolones, ciprofloxacin and levofloxacin, remained the most potent antibacterial agents.
Table 2.
In vitro activity of besifloxacin and comparator anti-infectives against bacterial pathogens from three clinical trialsa
| Organism | Minimum inhibitory concentration (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| BES | CIP | GAT | LVX | MXF | OFX | AZM | ||
| All isolates (n = 1324) | Range | 0.008–8 | ≤0.004–>8 | ≤0.004–>8 | ≤0.004–>8 | ≤0.004–>8 | 0.008–>8 | 0.008–>8 |
| MiC50 | 0.06 | 0.25 | 0.125 | 0.25 | 0.125 | 0.5 | 2 | |
| MiC90 | 0.25 | 2 | 0.5 | 1 | 0.5 | 2 | >8 | |
| Gram-positive (n = 886) | Range | 0.008–8 | 0.015–>8 | 0.008–>8 | 0.008–>8 | 0.008–>8 | 0.008–>8 | 0.008–>8 |
| MiC50 | 0.06 | 0.5 | 0.25 | 0.5 | 0.125 | 1 | 1 | |
| MiC90 | 0.25 | 4 | 1 | 2 | 0.5 | 4 | >8 | |
| Gram-negative (n = 438) | Range | 0.008–8 | ≤0.004–>8 | ≤0.004–8 | ≤0.004–8 | ≤0.004–8 | 0.015–>8 | 0.015–>8 |
| MiC50 | 0.03 | 0.015 | 0.015 | 0.03 | 0.03 | 0.03 | 2 | |
| MiC90 | 0.5 | 0.125 | 0.25 | 0.125 | 0.25 | 0.25 | >8 | |
| Aerococcus viridans (n = 10) | Range | 0.015–0.03 | 0.06–0.125 | 0.06–0.5 | 0.125–0.25 | 0.03–0.06 | 0.125–0.25 | 4–>8 |
| MiC50 | 0.03 | 0.06 | 0.25 | 0.125 | 0.03 | 0.25 | 4 | |
| MiC90 | 0.03 | 0.06 | 0.5 | 0.25 | 0.06 | 0.25 | 8 | |
| CDC coryneform group G (n = 29) | Range | 0.008–2 | 0.03–8 | 0.03–8 | 0.06–>8 | 0.03–>8 | 0.125–>8 | 0.06–>8 |
| MiC50 | 0.015 | 0.06 | 0.06 | 0.06 | 0.03 | 0.125 | 0.125 | |
| MiC90 | 0.125 | 0.5 | 0.5 | 1 | 0.25 | 2 | >8 | |
| Haemophilus influenzae | Range | 0.008–0.5 | ≤0.004–1 | ≤0.004–0.5 | ≤0.004–1 | 0.008–1 | 0.015–2 | 0.015–>8 |
| All (n = 344) | MiC50 | 0.03 | 0.015 | 0.015 | 0.03 | 0.03 | 0.03 | 2 |
| MiC90 | 0.06 | 0.015 | 0.03 | 0.03 | 0.06 | 0.06 | 4 | |
| BL-neg (n = 253) | Range | 0.008–0.5 | 0.008–1 | 0.008–0.5 | 0.008–1 | 0.008–1 | 0.015–2 | 0.015–>8 |
| MiC50 | 0.03 | 0.015 | 0.015 | 0.03 | 0.03 | 0.03 | 2 | |
| MiC90 | 0.06 | 0.015 | 0.03 | 0.03 | 0.06 | 0.06 | 4 | |
| BL-pos (n = 87) | Range | 0.008–0.06 | ≤0.004–0.06 | ≤0.004–0.06 | ≤0.004–0.06 | 0.008–0.125 | 0.015–0.125 | 0.125–4 |
| MiC50 | 0.03 | 0.015 | 0.015 | 0.03 | 0.03 | 0.03 | 2 | |
| MiC90 | 0.06 | 0.015 | 0.03 | 0.03 | 0.06 | 0.06 | 2 | |
| Moraxella catarrhalis | Range | 0.06–0.25 | 0.03–1 | 0.03–0.5 | 0.06–2 | 0.06–0.5 | 0.06–4 | 0.03–0.25 |
| All (n = 12) | MiC50 | 0.06 | 0.03 | 0.06 | 0.06 | 0.125 | 0.125 | 0.06 |
| MiC90 | 0.25 | 0.5 | 0.25 | 1 | 0.5 | 2 | 0.25 | |
| BL-pos (n = 11) | Range | 0.06–0.25 | 0.03–1 | 0.03–0.5 | 0.06–2 | 0.06–0.5 | 0.06–4 | 0.06–0.25 |
| MiC50 | 0.06 | 0.03 | 0.06 | 0.06 | 0.125 | 0.125 | 0.06 | |
| MiC90 | 0.25 | 0.5 | 0.25 | 1 | 0.5 | 2 | 0.25 | |
| Staphylococcus aureus | Range | 0.008–8 | 0.06–>8 | 0.03–>8 | 0.03–>8 | 0.03–>8 | 0.125–>8 | 0.06–>8 |
| All (n = 190) | MiC50 | 0.03 | 0.5 | 0.125 | 0.25 | 0.06 | 0.5 | 2 |
| MiC90 | 0.5 | >8 | 4 | 8 | 2 | >8 | >8 | |
| MSSA-CS (n = 144) | Range | 0.008–1 | 0.06–1 | 0.03–>8 | 0.03–0.5 | 0.03–0.25 | 0.125–1 | 0.06–>8 |
| MiC50 | 0.03 | 0.5 | 0.125 | 0.25 | 0.06 | 0.5 | 2 | |
| MiC90 | 0.06 | 0.5 | 0.25 | 0.25 | 0.125 | 0.5 | >8 | |
| MRSA-CS (n = 9) | Range | 0.03–0.06 | 0.25–0.5 | 0.06–0.25 | 0.25–0.5 | 0.06–0.06 | 0.25–1 | 2–>8 |
| MiC50 | 0.06 | 0.5 | 0.125 | 0.25 | 0.06 | 0.5 | >8 | |
| MSSA-CR (n = 17) | Range | 0.125–2 | 4–>8 | 0.25–>8 | 1–>8 | 0.25–>8 | 2–>8 | 2–>8 |
| MiC50 | 0.5 | >8 | 4 | 8 | 2 | >8 | >8 | |
| MiC90 | 2 | >8 | >8 | >8 | 8 | >8 | >8 | |
| MRSA-CR (n = 17) | Range | 0.125–8 | 4–>8 | 0.25–>8 | 1–>8 | 0.5–>8 | 2–>8 | 2–>8 |
| MiC50 | 0.5 | >8 | 4 | 8 | 4 | >8 | >8 | |
| MiC90 | 4 | >8 | >8 | >8 | >8 | >8 | >8 | |
| Staphylococcus epidermidis | Range | 0.03–4 | 0.125–>8 | 0.06–>8 | 0.125–>8 | 0.06–>8 | 0.25–>8 | 0.5–>8 |
| All (n = 111) | MiC50 | 0.06 | 0.25 | 0.125 | 0.25 | 0.125 | 0.5 | 1 |
| MiC90 | 0.5 | >8 | 2 | 8 | 4 | >8 | >8 | |
| MSSE-CS (n = 50) | Range | 0.03–0.25 | 0.125–1 | 0.125–0.5 | 0.125–0.5 | 0.06–0.25 | 0.25–1 | 0.5–>8 |
| MiC50 | 0.06 | 0.25 | 0.125 | 0.25 | 0.125 | 0.5 | 1 | |
| MiC90 | 0.06 | 0.25 | 0.25 | 0.25 | 0.125 | 0.5 | >8 | |
| MRSE-CS (n = 27) | Range | 0.03–0.06 | 0.125–0.5 | 0.06–0.5 | 0.125–0.25 | 0.06–0.25 | 0.25–0.5 | 0.5–>8 |
| MiC50 | 0.06 | 0.25 | 0.125 | 0.25 | 0.125 | 0.5 | >8 | |
| MiC90 | 0.06 | 0.25 | 0.25 | 0.25 | 0.125 | 0.5 | >8 | |
| MSSE-CR (n = 10) | Range | 0.5–2 | 8–>8 | 2–>8 | 4–>8 | 1–>8 | 4–>8 | 0.5–>8 |
| MiC50 | 0.5 | >8 | 2 | 8 | 4 | >8 | 1 | |
| MiC90 | 1 | >8 | 8 | >8 | 8 | >8 | >8 | |
| MRSE-CR (n = 24) | Range | 0.25–4 | 4–>8 | 1–>8 | 1–>8 | 1–>8 | 8–>8 | 1–>8 |
| MiC50 | 0.5 | 8 | 2 | 8 | 2 | 8 | >8 | |
| MiC90 | 4 | >8 | >8 | >8 | >8 | >8 | >8 | |
| Streptococcus mitis (n = 20) | Range | 0.06–0.25 | 0.25–4 | 0.25–1 | 0.5–2 | 0.06–0.5 | 1–4 | 0.06–8 |
| MiC50 | 0.125 | 1 | 0.5 | 1 | 0.125 | 2 | 2 | |
| MiC90 | 0.125 | 2 | 1 | 1 | 0.25 | 2 | 4 | |
| S. mitis group (n = 45) | Range | 0.03–1 | 0.06–>8 | 0.06–2 | 0.125–>8 | 0.03–2 | 0.25–>8 | 0.03–>8 |
| MiC50 | 0.125 | 1 | 0.5 | 1 | 0.125 | 2 | 2 | |
| MiC90 | 0.25 | 4 | 0.5 | 2 | 0.25 | 4 | 8 | |
| Streptococcus oralis (n = 18) | Range | 0.015–0.25 | 0.03–4 | 0.03–1 | 0.125–2 | 0.015–0.5 | 0.125–4 | 0.06–>8 |
| MiC50 | 0.125 | 2 | 0.5 | 1 | 0.25 | 2 | 4 | |
| MiC90 | 0.25 | 4 | 1 | 2 | 0.25 | 4 | >8 | |
| Streptococcus pneumoniae | Range | 0.03–0.25 | 0.125–>8 | 0.125–1 | 0.125–2 | 0.06–1 | 0.5–4 | 0.06–>8 |
| All (n = 302) | MiC50 | 0.06 | 0.5 | 0.25 | 0.5 | 0.125 | 1 | 0.125 |
| MiC90 | 0.125 | 1 | 0.5 | 1 | 0.125 | 2 | >8 | |
| PSSP (n = 220) | Range | 0.03–0.25 | 0.125–2 | 0.125–1 | 0.125–2 | 0.06–1 | 0.5–4 | 0.06–>8 |
| MiC50 | 0.06 | 0.5 | 0.25 | 0.5 | 0.125 | 1 | 0.125 | |
| MiC90 | 0.125 | 1 | 0.5 | 1 | 0.125 | 2 | 0.25 | |
| PISP (n = 71) | Range | 0.03–0.125 | 0.25–2 | 0.125–0.5 | 0.5–2 | 0.06–0.5 | 1–2 | 0.06–>8 |
| MiC50 | 0.06 | 0.5 | 0.25 | 0.5 | 0.125 | 1 | 8 | |
| MiC90 | 0.06 | 0.5 | 0.5 | 1 | 0.125 | 2 | >8 | |
| PRSP (n = 11) | Range | 0.03–0.125 | 0.25–>8 | 0.25–1 | 0.5–1 | 0.125–0.25 | 1–2 | 0.06–>8 |
| MiC50 | 0.06 | 1 | 0.5 | 1 | 0.125 | 2 | 8 | |
| MiC90 | 0.125 | 1 | 0.5 | 1 | 0.125 | 2 | >8 | |
| Unspeciated streptococci (n = 13) | Range | 0.015–0.25 | 0.03–4 | 0.03–1 | 0.125–2 | 0.015–0.5 | 0.125–4 | 0.125–>8 |
| MiC50 | 0.03 | 0.06 | 0.5 | 0.125 | 0.06 | 0.25 | 8 | |
| MiC90 | 0.125 | 1 | 1 | 1 | 0.5 | 2 | >8 | |
Note: Species isolated from ≥ 10 patients.
Abbreviations: BES, besifloxacin; CIP, ciprofloxacin; GAT, gatifloxacin; LVX, levofloxacin; MXF, moxifloxacin; OFX, ofloxacin; AZM, azithromycin; OXA, oxacillin; PEN, penicillin; BL-neg, β-lactamase negative; BL-pos, β-lactamase positive; MSSA-CS, methicillin-susceptible ciprofloxacin-susceptible S. aureus; MRSA-CS, methicillin-resistant ciprofloxacin-susceptible S. aureus; MSSA-CR, methicillin-susceptible ciprofloxacin-resistant S. aureus; MRSA-CR, methicillin-resistant ciprofloxacin-resistant S. aureus; MSSECS, methicillin-susceptible ciprofloxacin-susceptible S. epidermidis; MRSE-CS, methicillin-resistant ciprofloxacin-susceptible S. epidermidis; MSSE-CR, methicillin-susceptible ciprofloxacin-resistant S. epidermidis; MRSE-CR, methicillin-resistant ciprofloxacin-resistant S. epidermidis; PSSP, penicillin-susceptible S. pneumoniae; PISP, penicillin-intermediate S. pneumoniae; PRSP, penicillin-resistant S. pneumoniae.
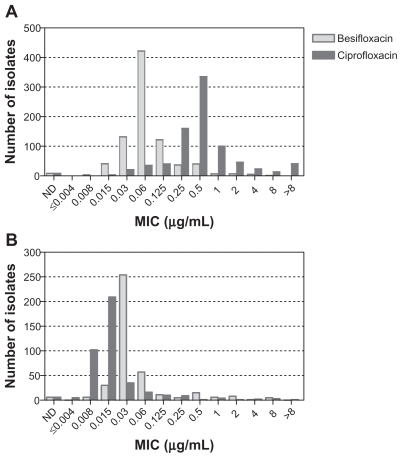
Figure 2 shows the MIC distributions for besifloxacin and ciprofloxacin for Gram-positive and Gram-negative isolates obtained from patients at US clinical sites. The 825 Gram-positive isolates among US isolates had MIC50/ MIC90 values of 0.06/0.25 μg/mL for besifloxacin and 0.5/2 μg/mL for ciprofloxacin. Many Gram-positive isolates had ciprofloxacin MICs ≥ 4 μg/mL, reflecting the ciprofloxacin resistance among staphylococcal isolates (discussed further below). The corresponding MIC50/ MIC90 values for the 404 Gram-negative isolates were 0.03/0.25 μg/mL for besifloxacin and 0.015/0.06 μg/mL for ciprofloxacin.
Figure 2.
Distribution of minimum inhibitory concentrations for besifloxacin (light gray) and ciprofloxacin (dark gray) for 825 Gram-positive (A) and 438 Gram-negative isolates from the US (B).
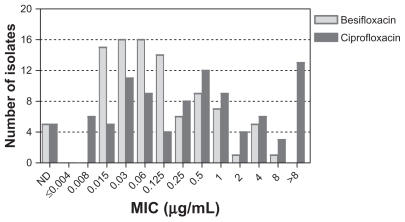
Figure 3 presents the MIC distributions for besifloxacin and ciprofloxacin for all isolates obtained from patients at Asian clinical sites, which included sites in India and the Philippines. While the MIC distributions for isolates obtained from US clinical sites had a distinct peak, MIC values for isolates obtained from Asian sites were more widely distributed, and ciprofloxacin MICs ≥ 4 μg/mL were more common, indicating that isolates from Asian sites were overall more resistant to ciprofloxacin compared with isolates from US sites. The increase in ciprofloxacin MIC90 values between isolates from US and Asian sites was noted overall for Gram-positive and Gram-negative bacteria and specifically for S. aureus and H. influenzae (discussed further below).
Figure 3.
Distribution of minimum inhibitory concentrations for besifloxacin (light gray) and ciprofloxacin (dark gray) for 95 isolates from Asia.
In vitro susceptibility of most prevalent genera
Of the H. influenzae isolates, 25.3% (87/344) were β-lactamase positive. As expected, H. influenzae MIC50/MIC90 values were low for all fluoroquinolones tested regardless of β-lactamase status. Although no fluoroquinolone-resistant isolates were recovered, two strains with elevated MIC values of 1 μg/mL for ciprofloxacin, levofloxacin, and moxifloxacin were identified. H. influenzae isolates from Asian clinical sites had higher MIC90 values for fluoroquinolones than those from US sites, with MIC90 value ranges of 0.25–1 μg/mL for isolates from Asian sites (n = 12) and 0.015–0.06 μg/mL for isolates from US sites (n = 332).
Overall, besifloxacin was the most active agent tested against all streptococcal isolates. Among S. pneumoniae isolates, 72.8% (220/302) were penicillin-susceptible, 23.5% (71/302) were penicillin-intermediate (PISP), and 3.6% (11/302) were penicillin-resistant (PRSP). Penicillin susceptibility/resistance did not influence fluoroquinolone MIC50/MIC90 values. Among PISP and PRSP isolates, the azithromycin MIC50 values were 8 μg/mL, and MIC90 values were >8 μg/mL, indicating that dual resistance to azithromycin and penicillin is not uncommon in S. pneumoniae. The MIC50/MIC90 values for each of the fluoroquinolones were similar against Streptococcus oralis, S. mitis, and S. mitis group isolates; besifloxacin was the most potent, followed by moxifloxacin, gatifloxacin, levofloxacin, ciprofloxacin, and ofloxacin.
Among S. aureus isolates, 75.8% (144/190) were methicillin-susceptible and ciprofloxacin-susceptible, 8.9% (17/190) were methicillin-susceptible and ciprofloxacin-resistant (MSSA-CR), 4.7% (9/190) were methicillin-resistant and ciprofloxacin-susceptible, 8.9% (17/190) were resistant to both antibacterials (MRSA-CR), and 1.6% (3/190) were ciprofloxacin-intermediate (two methicillin-susceptible and one methicillin-resistant). Based on MIC50/MIC90 values, besifloxacin was the most potent fluoroquinolone against all of these phenotypes. Against ciprofloxacin-resistant isolates of S. aureus, MIC50 and MIC90 values for besifloxacin were at least four-fold lower than for the next most active fluoroquinolone, moxifloxacin. MIC90 values for azithromycin were >8 μg/mL for all resistance phenotypes of S. aureus. Azithromycin resistance was especially prominent among MRSA isolates, with at least 50% of all isolates having MIC values >8 μg/mL. S. aureus isolates from US clinical sites had different MIC values compared with those from Asian sites. The MIC50 values for the fluoroquinolones for isolates from US clinical sites (n = 170) ranged from 0.03–0.5 μg/mL compared with 0.5 μg/mL to more than 8 μg/mL for isolates from Asian sites (n = 20). In contrast, the MIC90 value for oxacillin was higher in isolates from US clinical sites compared with Asian sites (>8 μg/mL and 0.5 μg/mL, respectively). Consistent with this finding, 13 of the 17 MSSA-CR isolates in the overall data set came from patients in Asia, whereas all 26 MRSA isolates were obtained from clinical sites in the US, including 17 isolates that were also ciprofloxacin-resistant.
Among S. epidermidis isolates, 45.0% (50/111) were methicillin-susceptible and ciprofloxacin-susceptible, while 24.3% (27/111) were methicillin-resistant and ciprofloxacinsusceptible. A further 9.0% (10/111) and 21.6% (24/111) of isolates were methicillin-susceptible, ciprofloxacin-resistant, and methicillin-resistant and ciprofloxacin-resistant (MRSE-CR). Besifloxacin MIC50 values were the lowest for the ciprofloxacin-resistant isolates (0.5 μg/mL) regardless of methicillin susceptibility, followed by gatifloxacin and moxifloxacin (2–4 μg/mL) and ciprofloxacin, levofloxacin, and ofloxacin (8 μg/mL to more than 8 μg/mL). The MIC90 values were ≥8 μg/mL for all fluoroquinolones except besifloxacin. Azithromycin MIC90 values were >8 μg/mL for all S. epidermidis isolates regardless of methicillin-resistant or ciprofloxacin-resistant phenotype.
To assess these relative potency differences against ciprofloxacin-resistant staphylococcal isolates further, any isolates with an MIC > 8 μg/mL for any of the fluoroquinolones tested in the initial analysis were retested at higher drug concentrations. Results of the retest are shown in Table 3. For MSSA-CR, besifloxacin showed a 4–128-fold greater potency compared with the other fluoroquinolones, while for MRSA-CR, besifloxacin showed a 16–128-fold greater potency compared with other fluoroquinolones. Likewise, for MRSE-CR, besifloxacin showed an 8–64-fold greater potency compared with other fluoroquinolones.
Table 3.
In vitro activity of besifloxacin and comparator fluoroquinolones against ciprofloxacin resistant Staphylococcus aureus and Staphylococcus epidermidis: Results of the expanded range retest
| Organism | Fluoroquinolone | MIC (μg/mL) | MIC90 versus besifloxacin | ||
|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | |||
| MSSA–CR (n = 14) | Besifloxacin | 0.5–2 | 0.5 | 1 | – |
| Moxifloxacin | 2–16 | 2 | 4 | 4× | |
| Gatifloxacin | 2–16 | 4 | 8 | 8× | |
| Levofloxacin | 4–128 | 8 | 16 | 16× | |
| Ciprofloxacin | 16–256 | 32 | 128 | 128× | |
| MRSA–CR (n = 15) | Besifloxacin | 0.5–16 | 1 | 4 | – |
| Moxifloxacin | 2–128 | 4 | 64 | 16× | |
| Gatifloxacin | 2–256 | 8 | 64 | 16× | |
| Levofloxacin | 4–512 | 16 | 512 | 128× | |
| Ciprofloxacin | 16–256 | 128 | 256 | 64× | |
| MSSE–CR (n = 9)a | Besifloxacin | 0.5 | 0.5 | ||
| Moxifloxacin | 2–4 | 4 | |||
| Gatifloxacin | 2–4 | 4 | |||
| Levofloxacin | 8–16 | 16 | |||
| Ciprofloxacin | 8–128 | 64 | |||
| MRSE–CR (n = 13) | Besifloxacin | 0.5–8 | 0.5 | 4 | – |
| Moxifloxacin | 2–32 | 4 | 32 | 8× | |
| Gatifloxacin | 2–64 | 4 | 64 | 16× | |
| Levofloxacin | 4–256 | 16 | 256 | 64× | |
| Ciprofloxacin | 8–64 | 64 | 64 | 16× | |
Note: Due to the limited isolates, only the MIC50 value is given.
Abbreviations: MSSA-CR, methicillin-susceptible ciprofloxacin-resistant Staphylococcus aureus; MRSA-CR, methicillin-resistant ciprofloxacin-resistant S. aureus; MSSECR, methicillin-susceptible ciprofloxacin-resistant Staphylococcus epidermidis; MRSE-CR, methicillin-resistant ciprofloxacin-resistant S. epidermidis; MIC, minimum inhibitory concentration.
Overall, besifloxacin was the most potent antibacterial agent tested against corynebacteria, while ofloxacin was the least potent fluoroquinolone. The MIC50/ MIC90 values for besifloxacin against CDC coryneform group G were 0.015 μg/mL and 0.125 μg/mL. In contrast, the comparators, ie, moxifloxacin, gatifloxacin, ciprofloxacin, and levofloxacin, had MIC50 values that were 2–4-fold higher and MIC90 values that were 2–8-fold higher.
In vitro susceptibility of other species of ophthalmic interest
Table 4 presents susceptibility data for less frequently isolated ocular pathogens of particular interest in ophthalmology, namely Neisseria spp, Pseudomonas aeruginosa, and Serratia marcescens. Eight P. aeruginosa isolates were obtained in the three clinical studies. Ciprofloxacin was the most active fluoroquinolone against P. aeruginosa, with MIC values ranging from 0.125 μg/mL to 1 μg/mL. Less active were besifloxacin (MIC range 1–4 μg/mL) and moxifloxacin (MIC range 1–8 μg/mL). The MIC values for Neisseria spp (n = 7) varied from 0.008 μg/mL and 0.25 μg/mL for besifloxacin to ≤0.004 μg/mL and 2 μg/mL for ciprofloxacin. Nine S. marcescens isolates were obtained in the three clinical studies. Ciprofloxacin was the most active fluoroquinolone against S. marcescens, with MIC values ranging from 0.03 to 0.5 μg/mL.
Table 4.
In vitro activity of besifloxacin and comparator fluoroquinolones against pathogens of ophthalmic interest
| MIC (μg/mL) | |||
|---|---|---|---|
| Organism | Fluoroquinolone | Range | MIC50 |
| Neisseria spp (n = 7)a | Besifloxacin | 0.008–0.25 | 0.015 |
| Moxifloxacin | 0.008–1 | 0.015 | |
| Gatifloxacin | 0.008–0. 5 | 0.25 | |
| Levofloxacin | 0.008–2 | 0.015 | |
| Ciprofloxacin | ≤0.004–2 | 0.03 | |
| Pseudomonas aeruginosa (n = 8) | Besifloxacin | 1–4 | 2 |
| Moxifloxacin | 1–8 | 2 | |
| Gatifloxacin | 0.5–4 | 1 | |
| Levofloxacin | 0.5–2 | 1 | |
| Ciprofloxacin | 0.125–1 | 0.25 | |
| Serratia marcescens (n = 9) | Besifloxacin | 0.125–1 | 0.5 |
| Moxifloxacin | 0.125–4 | 0.5 | |
| Gatifloxacin | 0.06–1 | 0.25 | |
| Levofloxacin | 0.06–0.5 | 0.25 | |
| Ciprofloxacin | 0.03–0.5 | 0.125 | |
Note: Includes Neisseria meningitidis (n = 3), Neisseria gonorrhoeae (n = 2), Neisseria sicca (n = 1), Neisseria subflavia (n = 1).
Abbreviation: MIC, minimum inhibitory concentration.
Discussion
The primary objective of this study was to report on the bacterial pathogen distribution across three clinical trials of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis and to report on the in vitro antibacterial susceptibility of these pathogens. The clinical antimicrobial efficacy of besifloxacin integrated across these three clinical trials is described in the companion paper by Morris et al.15 A total of 1324 bacterial pathogens were isolated across these studies from 1041 patients with culture-confirmed bacterial conjunctivitis, with H. influenzae, S. pneumoniae, S. aureus, and S. epidermidis being the most prevalent species identified. Other species of Corynebacterium, Streptococcus, and Staphylococcus were less frequently isolated, while only a few members of the Acinetobacter, Enterobacteriaceae, Moraxella, Neisseria, and Pseudomonas spp were recovered. Consistent with previous reports,4,5 2.7% of eyes at baseline yielding bacterial isolates were coinfected with virus, primarily adenovirus, indicating that bacterial and viral infections can occur together.
Previous studies on the etiology of bacterial conjunctivitis reported the same or similar bacterial pathogens, although sometimes with different frequencies.1,7,23,24 These differences might be due to several factors, including bacterial culture conditions, nomenclature, detection methods, patient age and geographic origin, as well as the threshold used to characterize an isolate as pathogenic. Sampling the surface of the eyes of healthy volunteers has shown that most conjunctivae are colonized by various bacterial species, such as staphylococci and corynebacteria.1 Cagle based his definition of a conjunctivitis pathogen on the number of bacteria isolated from a patient relative to the number of bacteria of the same species isolated from healthy subjects.16 If the number of bacteria from a patient exceeded a predetermined species-specific threshold level, then the isolate was considered to be the cause of the disease. The Cagle criteria were applied in the current analysis.
The present study shows differences in the relative pathogen distribution of bacterial conjunctivitis by age. In both the 1–2-year and 3–17-year age groups, H. influenzae was the most frequently isolated pathogen, followed by S. pneumoniae and other streptococci. In patients 18–64 years of age, the most common pathogens were S. pneumoniae, H. influenzae, and S. aureus. In patients 65 years and older, S. aureus was the most prevalent isolate, followed by corynebacteria and H. influenzae. In general, the contribution of H. influenzae, S. pneumoniae, other streptococci, and Moraxella catarrhalis to the number of bacterial conjunctivitis isolates decreased with increasing patient age, while staphylococci, corynebacteria, and Gram-negative species (Enterobacteriaceae, Neisseria spp, Pseudomonas spp) increased in prevalence with increasing patient age. These results are consistent with other studies.7,23,25,26
The overall species contribution was similar between the US and Asian clinical sites, with the exception of S. pneumoniae, which was far less prevalent among isolates from Asian sites. Mahajan et al reported on the etiology of bacterial conjunctivitis in India and likewise found a low prevalence of S. pneumoniae isolates, specifically 7.5%.24 Isolates obtained from clinical sites in Asia and the US also differed in their antibacterial resistance profiles, most notably for S. aureus and H. influenzae. For both species, MIC values for all of the fluoroquinolones were higher for isolates obtained from Asian clinical sites compared with US sites, while for S. aureus oxacillin MIC values were higher in isolates obtained from US sites compared with Asian sites.
Approximately one-fourth of H. influenzae and S. pneumoniae isolates were β-lactamase-positive and penicillin-intermediate-resistant, respectively, a trend which has been reported previously.25,27 Cavuoto et al and Adebayo et al recently reported an increase in methicillin resistance among S. aureus isolates from bacterial conjunctivitis patients.23,28 Similarly, in this study, 13.7% of S. aureus isolates and 45.9% of S. epidermidis isolates were methicillin-resistant, and, of these, a further 65.4% and 47.1% were also ciprofloxacin-resistant. In addition, many of the methicillin-resistant and ciprofloxacin-resistant isolates were also resistant to azithromycin, indicating that resistance to two or three antibacterial agents is not uncommon. This is consistent with previous studies of ocular MRSA isolates in which we found similar multidrug resistance trends among isolates characterized as hospital-associated as well as community-acquired.29 While multidrug resistance among ocular isolates has been reported in various surveillance studies,30–32 to our knowledge this finding represents the largest and most recent analysis of multidrug resistance observed in prospective and controlled clinical studies of bacterial conjunctivitis. Thus, while the spectrum of causative pathogens associated with bacterial conjunctivitis has not changed, multidrug resistance among common conjunctivitis pathogens is evolving.
The in vitro potency of besifloxacin was similar to or exceeded that of comparator antibacterials. Against Gram-positive isolates, besifloxacin was the most potent drug, followed by moxifloxacin, gatifloxacin, levofloxacin, and ciprofloxacin. Against Gram-negative bacteria, ciprofloxacin was the most potent antibacterial, while the potency of besifloxacin was similar to that of moxifloxacin. In agreement with previous reports,32–35 besifloxacin’s potency against ciprofloxacin-resistant staphylococcal isolates far exceeded that of other ophthalmic fluoroquinolones. Results of susceptibility retests indicated that besifloxacin was 4–128-fold more potent against ciprofloxacin-resistant MRSA and MRSE compared with other fluoroquinolones. Thus, although the methicillin-resistance phenotype does not affect fluoroquinolone relative potency, ciprofloxacin resistance, which is often concurrent with methicillin resistance, did. Silverstein et al recently reported that the in vitro potency of besifloxacin was similar to that of vancomycin against staphylococcal isolates, including ciprofloxacin-resistant MRSA.36 Vancomycin is often used in the treatment of ocular MRSA infections.28,37,38
While the superior in vitro activity of besifloxacin against drug-resistant staphylococcal isolates is notable, the clinical relevance of these in vitro results remains to be shown. Topical administration of ocular antibiotics results in tear and conjunctival tissue concentrations often several-fold higher than the MIC, even if the latter is elevated due to development of resistance, raising the possibility that some antibacterials may be clinically effective even against bacterial strains with increased MICs. Nevertheless, the vitro potency of besifloxacin in conjunction with the favorable pharmacokinetic profile at the ocular surface39,40 could provide a clinical advantage. After a single dose, besifloxacin exposure on the ocular surface results in Cmax/MIC and AUC0–24/MIC ratios that are well above the generally accepted pharmacodynamic ratios required for fluoroquinolone efficacy (ie, Cmax/MIC ≥ 10 and AUC0–24/MIC ≥ 30–50 for Gram-positive bacteria or ≥100–125 for Gram-negative bacteria)41–43 even for drug-resistant staphylococcal isolates.
In summary, while the spectrum of causative pathogens associated with bacterial conjunctivitis has not changed, the incidence of resistance of these organisms to antibacterial agents has been increasing.23,28,31,32 Thus, there is a need for the development of novel anti-infective agents with improved potency and activity against drug-resistant pathogens. In this integrated data analysis, besifloxacin, a novel chlorofluoroquinolone, demonstrated broad-spectrum in vitro activity against the causative agents of bacterial conjunctivitis, with potent activity against multidrug-resistant staphylococcal isolates.
Acknowledgment
Species identification and antibacterial susceptibility testing were performed by Covance Laboratory Services Inc, Indianapolis, IN.
Footnotes
Disclosure
The authors were employees of Bausch & Lomb during the conduct of this analysis.
References
- 1.Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86(1):5–17. doi: 10.1111/j.1600-0420.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 2.Diamant JI, Hwang DG. Therapy for bacterial conjunctivitis. Ophthalmol Clin North Am. 1999;12(1):15–20. [Google Scholar]
- 3.Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006;2:CD001211. doi: 10.1002/14651858.CD001211.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Rose PW, Harnden A, Brueggemann AB, et al. Chloramphenicol treatment for acute infective conjunctivitis in children in primary care: A randomized double-blind placebo-controlled trial. Lancet. 2005;366(9479):37–43. doi: 10.1016/S0140-6736(05)66709-8. [DOI] [PubMed] [Google Scholar]
- 5.Gigliotti F, Williams WT, Hayden FG, et al. Etiology of acute conjunctivitis in children. J Pediatr. 1981;98(4):531–536. doi: 10.1016/s0022-3476(81)80754-8. [DOI] [PubMed] [Google Scholar]
- 6.Brook I, Pettit TH, Martin WJ, Finegold SM. Anaerobic and aerobic bacteriology of acute conjunctivitis. Ann Ophthalmol. 1979;11(3):389–393. [PubMed] [Google Scholar]
- 7.Tarabishy AB, Jeng BH. Bacterial conjunctivitis: A review for internists. Cleve Clin J Med. 2008;75(7):507–512. doi: 10.3949/ccjm.75.7.507. [DOI] [PubMed] [Google Scholar]
- 8.Cambeau E, Matrat S, Pan X-S, et al. Target specificity of the new fluoroquinolone besifloxacin in Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli. J Antimicrob Chemother. 2009;63(3):443–450. doi: 10.1093/jac/dkn528. [DOI] [PubMed] [Google Scholar]
- 9.Haas W, Pillar CM, Zurenko GE, Lee JC, Brunner LS, Morris TW. Besifloxacin, a novel fluoroquinolone, has broad-spectrum in vitro activity against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 2009;53(8):3552–3560. doi: 10.1128/AAC.00418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas W, Pillar CM, Hesje CK, Sanfilippo CM, Morris TW. Bactericidal activity of besifloxacin against staphylococci, Streptococcus pneumoniae and Haemophilus influenzae. J Antimicrob Chemother. 2010;65(7):1441–1447. doi: 10.1093/jac/dkq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas W, Pillar CM, Hesje CK, Sanfilippo CM, Morris TW. In vitro time-kill experiments with besifloxacin and gatifloxacin in the absence and presence of benzalkonium chloride. J Antimicrob Chemother. 2011;66(4):840–844. doi: 10.1093/jac/dkq531. [DOI] [PubMed] [Google Scholar]
- 12.Karpecki P, Depaolis M, Hunter JA, et al. Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: A multicenter, prospective, randomized, double-masked, vehicle-controlled, 5-day efficacy and safety study. Clin Ther. 2009;31(3):514–526. doi: 10.1016/j.clinthera.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Tepedino ME, Heller WH, Usner DW, et al. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr Med Res Opin. 2009;25(5):1159–1169. doi: 10.1185/03007990902837919. [DOI] [PubMed] [Google Scholar]
- 14.McDonald MB, Protzko EE, Brunner LS, et al. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. Ophthalmology. 2009;116(9):1615–1623. doi: 10.1016/j.ophtha.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Morris TW, Gearinger LS, Usner DW, et al. Integrated analysis of three bacterial conjunctivitis trials of besifloxacin ophthalmic suspension, 0.6%: microbiological eradication outcomes. Clin Ophthalmol. 2011 doi: 10.2147/OPTH.S23518. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cagle G, Davis S, Rosenthal A, Smith J. Topical tobramycin and gentamicin sulfate in the treatment of ocular infections: Multicenter study. Curr Eye Res. 1981;1(9):523–534. doi: 10.3109/02713688109069178. [DOI] [PubMed] [Google Scholar]
- 17.Leibowitz HM. Antibacterial effectiveness of ciprofloxacin 0.3% ophthalmic solution in the treatment of bacterial conjunctivitis. Am J Ophthalmol. 1991;112(Suppl 4):29S–33S. [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. CLSI document M07-A6. Sixth Edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2003. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. CLSI document M07-A7. Seventh Edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. CLSI document M100-S14. Wayne, PA: Clinical and Laboratory Standards Institute; 2004. Performance Standards for Antimicrobial Susceptibility Testing; Fourteenth Informational Supplement. [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. CLSI document M100-S16. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. Performance Standards for Antimicrobial Susceptibility Testing; Sixteenth Informational Supplement. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. CLSI document: M100-S21. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-first Informational Supplement. [Google Scholar]
- 23.Cavuoto K, Zutshi D, Karp CL, Miller D, Feuer W. Update on bacterial conjunctivitis in South Florida. Ophthalmology. 2008;115(1):51–56. doi: 10.1016/j.ophtha.2007.03.076. [DOI] [PubMed] [Google Scholar]
- 24.Mahajan VM. Acute bacterial infections of the eye: Their aetiology and treatment. Br J Ophthalmol. 1983;67(3):191–194. doi: 10.1136/bjo.67.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buznach N, Dagan R, Greenberg D. Clinical and bacterial characteristics of acute bacterial conjunctivitis in children in the antibiotic resistance era. Pediatr Infect Dis J. 2005;24(9):823–828. doi: 10.1097/01.inf.0000178066.24569.98. [DOI] [PubMed] [Google Scholar]
- 26.Hautala N, Koskela M, Hautala T. Major age group-specific differences in conjunctival bacteria and evolution of antimicrobial resistance revealed by laboratory data surveillance. Curr Eye Res. 2008;33(11):907–911. doi: 10.1080/02713680802475338. [DOI] [PubMed] [Google Scholar]
- 27.Block SL, Hedrick J, Tyler R, et al. Increasing bacterial resistance in pediatric acute conjunctivitis (1997–1998) Antimicrob Agents Chemother. 2000;44(6):1650–1654. doi: 10.1128/aac.44.6.1650-1654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adebayo A, Parikh JG, McCormick SA, et al. Shifting trends in in vitro antibiotic susceptibilities for common bacterial isolates in the last decade at the New York Eye and Ear Infirmary. Graefes Arch Clin Exp Ophthalmol. 2011;249(1):111–119. doi: 10.1007/s00417-010-1426-6. [DOI] [PubMed] [Google Scholar]
- 29.Hesje CK, Sanfilippo CM, Haas W, Morris TW. Molecular epidemiology of methicillin resistant and methicillin susceptible Staphylococcus aureus isolated from the eye. Curr Eye Res. 2011;36(2):94–102. doi: 10.3109/02713683.2010.534229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: Nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008;145(6):951–958. doi: 10.1016/j.ajo.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 31.Pillar CM, Torres MK, Sahm DF. Ten year trends and current activity profile of fluoroquinolones among ocular isolates of S. aureus and S. epidermidis – Results from the TSN surveillance. Presented at the annual meeting of the Association for Research in Vision and Ophthalmology; 2010 May 2–6; Fort Lauderdale, FL. [Google Scholar]
- 32.Haas W, Pillar CM, Torres M, Morris TW, Sahm DF. Monitoring antibiotic resistance in ocular microorganisms: Results from the ARMOR 2009 surveillance study. Am J Ophthalmol. 2011 doi: 10.1016/j.ajo.2011.03.010. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 33.Haas W, Zurenko GE, Lee J, Pillar C, Brunner LS, Morris TW. Activity of besifloxacin and comparators against ciprofloxacin resistant Staphylococcus aureus and Staphylococcus epidermidis ocular isolates from 2005–2008. Presented at the Third Congress of the Federation of European Microbiologists Microbiology Congress; June 28–July 2, 2009; Gothenberg, Sweden. [Google Scholar]
- 34.Miller D. In vitro evaluation of besifloxacin against ciprofloxacin and moxifloxacin resistant/susceptible staphylococcal isolates from South Florida. Presented at the Fifth International Conference on Ocular Infections; February 18–21, 2010; Palm Beach, FL. [Google Scholar]
- 35.Chang JS, Flynn HW, Jr, Miller D, Alfonso EC. Evaluation of in vitro effectiveness of besifloxacin against Staphylococcus epidermidis. Presented at the annual meeting of the Association for Research in Vision and Ophthalmology; May 2–6, 2010; Fort Lauderdale, FL. [Google Scholar]
- 36.Silverstein BE, Allaire C, Bateman KM, Gearinger LS, Morris TW, Comstock TL. Efficacy and tolerability of besifloxacin 0.6% ophthalmic suspension administered twice daily for three days in the treatment of bacterial conjunctivitis: A multicenter, double-masked, vehicle controlled, parallel-group study in adults and children. Clin Ther. 2011;33(1):13–26. doi: 10.1016/j.clinthera.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Freidlin J, Acharya N, Lietman TM, Cevallos V, Whitcher JP, Margolis TP. Spectrum of eye disease caused by methicillin-resistant Staphylococcus aureus. Am J Ophthlamol. 2007;144(2):313–315. doi: 10.1016/j.ajo.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Blomquist HP. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:322–345. [PMC free article] [PubMed] [Google Scholar]
- 39.Proksch JW, Granvil CP, Mermet-Siou R, et al. Ocular pharmacokinetics of besifloxacin following topical administration to rabbits, monkeys and humans. J Ocul Pharmacol Ther. 2009;25(4):335–344. doi: 10.1089/jop.2008.0116. [DOI] [PubMed] [Google Scholar]
- 40.Torkildsen G, Proksch JW, Shapiro A, Lynch SK, Comstock TL. Concentrations of besifloxacin, gatifloxacin, and moxifloxacin in human conjunctiva after topical ocular administration. Clin Ophthalmol. 2010;(4):331–341. doi: 10.2147/opth.s9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright DH, Brown GH, Peterson ML, Rotshafer JC. Application of fluoroquinolone pharmacodynamics. J Antimicrob Chemother. 2000;46(5):669–683. doi: 10.1093/jac/46.5.669. [DOI] [PubMed] [Google Scholar]
- 42.Allen GP, Kaatz GW, Rybak MJ. In vitro activities of mutant prevention concentration-targeted concentrations of fluoroquinolones against Staphylococcus aureus and in a pharmacodynamic model. Int J Antimicrob Agents. 2004;24(2):150–160. doi: 10.1016/j.ijantimicag.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Metzler K, Hansen GM, Hedlin P, Harding E, Drlica K, Blondeau JM. Comparison of minimal inhibitory and mutant prevention concentrations of 4 fluoroquinolones against clinical isolates of methicillin-susceptible and -resistant Staphylococcus aureus. Int J Antimicrob Agents. 2004;24(2):161–167. doi: 10.1016/j.ijantimicag.2004.02.021. [DOI] [PubMed] [Google Scholar]