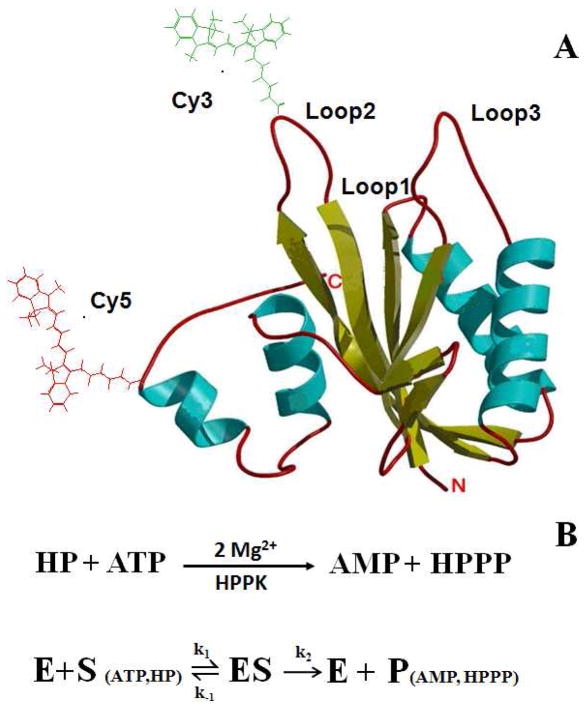
Figure 1.
(A) Crystal Structure of the Apo HPPK. The cyan spirals represent the α helices and the yellow arrows are the β strands. The loops are shown in red pipes. Amino acid residue 48 and 151 are labeled with FRET dye pair Cy3 and Cy5, respectively. The drawing is derived from the Protein Data Bank (1HKA). (B) Enzymatic reaction of HPPK-catalyzed pyrophosphoryl transfer. The dynamic processes involve (1) the binding of two substrates (ATP and HP) to the enzyme (E) to form the enzyme-substrate complex (ES); (2) the enzymatic turnover of the enzyme-substrate complex and the release of products (P).