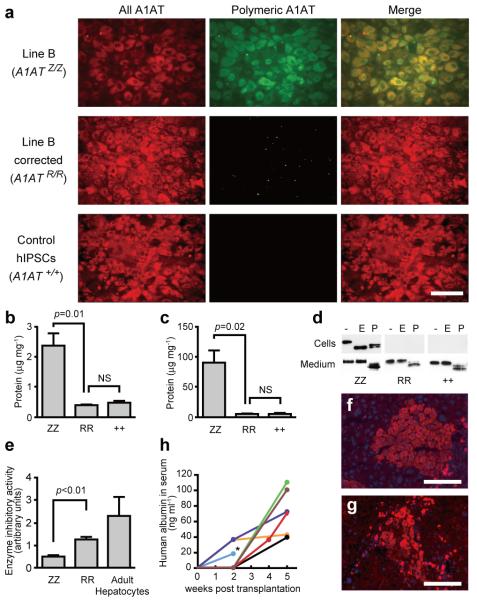
Figure 3. Functional analysis of restored A1AT in c-hIPSCs-derived hepatocyte-like cells.
a, Immunofluorescence showing the absence of polymeric A1AT protein in hepatocyte-like cells generated from c-hIPSCs. All forms of A1AT (left panels) and misfolded polymeric A1AT (middle panels). b, c, ELISA to assess the intracellular (b) and secreted (c) levels of polymeric A1AT protein in hepatocyte-like cells derived from A1ATD-hIPSCs (ZZ), c-hIPSCs (RR) and control hIPSCs (++). d, Endoglycosidase H (E) and peptide:N-glycosidase (P) digestion of A1AT immunoprecipitated from uncorrected (ZZ), corrected (RR) and control (++) hIPSC-derived hepatocyte-like cells (upper panels) and corresponding culture medium (lower panels). e, Chymotrypsin ELISA showing that corrected cells (RR) have A1AT enzymatic inhibitory activity that is superior to uncorrected cells (ZZ) and close to adult hepatocytes. f, g, Immunofluorescence of transplanted liver sections detecting human albumin (f) and A1AT (g). DNA was counterstained with DAPI. h, ELISA read-out of human albumin in the mouse serum longitudinally followed for each mouse. Asterisk, the mouse was subjected to histology analysis. Scale bars, 100 μm. Data in b, c and e are shown as mean ± s.d. (n=3). Student’s t-test was performed. NS, not significant.